Airway hyperresponsiveness, remodeling, and smooth muscle mass: right answer, wrong reason?
- PMID: 17463392
- PMCID: PMC1994228
- DOI: 10.1165/rcmb.2006-0418OC
Airway hyperresponsiveness, remodeling, and smooth muscle mass: right answer, wrong reason?
Abstract
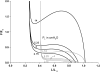
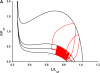
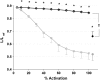
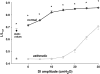
We quantified the effects of airway wall remodeling upon airway smooth muscle (ASM) shortening. Isolated ASM from sheep was attached to a servo-controller that applied a physiologic load. This load could be altered to reflect specified changes of airway wall geometry, elasticity, parenchymal tethering, transpulmonary pressure (P(L)), and fluctuations in P(L) associated with breathing. Starting at a reference length (L(ref)), ASM was stimulated with acetlycholine and held at constant P(L) of 4 cm H(2)O for 2 h. When all compartments were thickened to simulate the asthmatic airway but P(L) was held fixed, ASM shortened much more than that in the normal airway (to 0.52 L(ref) versus 0.66 L(ref)). When breathing with deep inspirations (DIs) was initiated, within the first three DIs the ASM in the normal airway lengthened to 0.84 L(ref), whereas that in the asthmatic airway remained stuck at 0.53 L(ref). Thickening of the smooth muscle layer alone produced the greatest muscle shortening (to 0.47 L(ref)) when compared with thickening of only submucosal (to 0.67 L(ref)) or only adventitial (to 0.62 L(ref)) compartments. With increased ASM mass, the ASM failed to lengthen in response to DIs, whereas in the airway with thickened submucosal and adventitial layers ASM lengthened dramatically (to 0.83 L(ref)). These findings confirm the long-held conclusion that increased muscle mass is the functionally dominant derangement, but mechanisms accounting for this conclusion differ dramatically from those previously presumed. Furthermore, increased ASM mass explained both hyperresponsiveness and the failure of a DI to relax the asthmatic airway.
Figures









References
-
- Woolcock AJ, Peat JK. Epidemiology of bronchial hyperresponsiveness. Clin Rev Allergy 1989;7:245–256. - PubMed
-
- McParland BE, Macklem PT, Pare PD. Airway wall remodeling: friend or foe? J Appl Physiol 2003;95:426–434. - PubMed
-
- Macklem PT. Mechanical factors determining maximum bronchoconstriction. Eur Respir J Suppl 1989;6:516s–519s. - PubMed
-
- Sterk PJ, Bel EH. Bronchial hyperresponsiveness: the need for a distinction between hypersensitivity and excessive airway narrowing. Eur Respir J 1989;2:267–274. - PubMed
-
- Bai TR, Knight DA. Structural changes in the airways in asthma: observations and consequences. Clin Sci (Lond) 2005;108:463–477. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

