Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5' elements
- PMID: 17463395
- PMCID: PMC1994232
- DOI: 10.1165/rcmb.2005-0460OC
Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5' elements
Abstract
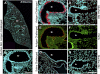
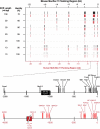
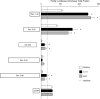
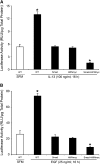
Mucus hypersecretion contributes to morbidity and mortality in many obstructive lung diseases. Gel-forming mucins are the chief glycoprotein components of airway mucus, and elevated expression of these during mucous metaplasia precedes the hypersecretory phenotype. Five orthologous genes (MUC2, MUC5AC, MUC5B, MUC6, and MUC19) encode the mammalian gel-forming mucin family, and several have been implicated in asthma, cystic fibrosis, and chronic obstructive pulmonary disease pathologies. However, in the absence of a comprehensive analysis, their relative contributions remain unclear. Here, we assess the expression of the entire gel-forming mucin gene family in allergic mouse airways and show that Muc5ac is the predominant gel-forming mucin induced. We previously showed that the induction of mucous metaplasia in ovalbumin-sensitized and -challenged mouse lungs occurs within bronchial Clara cells. The temporal induction and localization of Muc5ac transcripts correlate with the induced expression and localization of mucin glycoproteins in bronchial airways. To better understand the tight regulation of Muc5ac expression, we analyzed all available 5'-flanking sequences of mammalian MUC5AC orthologs and identified evolutionarily conserved regions within domains proximal to the mRNA coding region. Analysis of luciferase reporter gene activity in a mouse transformed Clara cell line demonstrates that this region possesses strong promoter activity and harbors multiple conserved transcription factor-binding motifs. In particular, SMAD4 and HIF-1alpha bind to the promoter, and mutation of their recognition motifs abolishes promoter function. In conclusion, Muc5ac expression is the central event in antigen-induced mucous metaplasia, and phylogenetically conserved 5' noncoding domains control its regulation.
Figures








References
-
- Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med 1996;154:1868–1902. - PubMed
-
- Evans CM, Agrawal A, Dickey BF. Strategies to reduce excessive mucus secretion in airway inflammation. Eissa T, Huston D, editors. Therapeutic targets of airway inflammation. New York: Marcel Dekker; 2002.
-
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of clara cells in normal human airway epithelium. Am J Respir Crit Care Med 1999;159:1585–1591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

