The outer membrane secretin PilQ from Neisseria meningitidis binds DNA
- PMID: 17464074
- PMCID: PMC2884949
- DOI: 10.1099/mic.0.2006/004200-0
The outer membrane secretin PilQ from Neisseria meningitidis binds DNA
Abstract
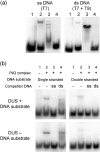
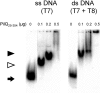
Neisseria meningitidis is naturally competent for transformation throughout its growth cycle. Transformation in neisserial species is coupled to the expression of type IV pili, which are present on the cell surface as bundled filamentous appendages, and are assembled, extruded and retracted by the pilus biogenesis components. During the initial phase of the transformation process, binding and uptake of DNA takes place with entry through a presumed outer-membrane channel into the periplasm. This study showed that DNA associates only weakly with purified pili, but binds significantly to the PilQ complex isolated directly from meningococcal membranes. By assessing the DNA-binding activity of the native complex PilQ, as well as recombinant truncated PilQ monomers, it was shown that the N-terminal region of PilQ is involved in the interaction with DNA. It was evident that the binding of ssDNA to PilQ had a higher affinity than the binding of dsDNA. The binding of DNA to PilQ did not, however, depend on the presence of the neisserial DNA-uptake sequence. It is suggested that transforming DNA is introduced into the cell through the outer-membrane channel formed by the PilQ complex, and that DNA uptake occurs by non-specific introduction of DNA coupled to pilus retraction, followed by presentation to DNA-binding component(s), including PilQ.
Figures



References
-
- Aas, F. E., Wolfgang, M., Frye, S., Dunham, S., Lovold, C. & Koomey, M. (2002). Competence for natural transformation in Neisseria gonorrhoeae: components of DNA binding and uptake linked to type IV pilus expression. Mol Microbiol 46, 749–760. - PubMed
-
- Bitter, W., Koster, M., Latijnhouwers, M., de Cock, H. & Tommassen, J. (1998). Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol 27, 209–219. - PubMed
-
- Bøvre, K., Bergan, T. & Froholm, L. O. (1970). Electron microscopical and serological characteristics associated with colony type in Moraxella nonliquefaciens. Acta Pathol Microbiol Scand [B] Microbiol Immunol 78, 765–779. - PubMed
-
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

