Fetal spina bifida in a mouse model: loss of neural function in utero
- PMID: 17465388
- PMCID: PMC3651953
- DOI: 10.3171/ped.2007.106.3.213
Fetal spina bifida in a mouse model: loss of neural function in utero
Abstract
Object: The devastating neurological deficit associated with myelomeningocele has previously been assumed to be a direct and inevitable consequence of the primary malformation-failure of neural tube closure. An alternative view is that secondary damage to the pathologically exposed spinal cord tissue in utero is responsible for the neurological deficiency. If the latter mechanism were shown to be correct, it would provide an objective rationale for the performance of in utero surgery for myelomeningocele, because coverage of the exposed spinal cord could be expected to alleviate or perhaps prevent neurodegeneration. To examine this question, the authors studied the development of neuronal connections and neurological function of mice during fetal and neonatal stages in a genetic model of exposed lumbosacral spina bifida.
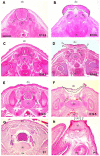
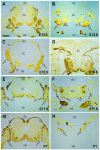
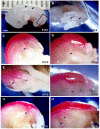
Methods: The persistently exposed spinal cord of mouse fetuses carrying both curly tail and loop-tail mutations exhibited essentially normal anatomical and functional hallmarks of development during early gestation (embryonic Days 13.5-16.5), including sensory and motor projections to and from the cord. A significant proportion of fetuses with spina bifida at early gestation exhibited sensorimotor function identical to that seen in age-matched healthy controls. However, at later gestational stages, increasing neurodegeneration within the spina bifida lesion was detected, which was paralleled by a progressive loss of neurological function.
Conclusions: These findings provide support for the hypothesis that neurological deficit in human myelomeningocele arises following secondary neural tissue destruction and loss of function during pregnancy.
Figures
Comment in
-
Fetal spina bifida.J Neurosurg. 2007 Mar;106(3 Suppl):211-2; discussion 212. doi: 10.3171/ped.2007.106.3.211. J Neurosurg. 2007. PMID: 17465387 No abstract available.
References
-
- Adzick NS, Harrison MR. Fetal surgical therapy. Lancet. 1994;343:897–902. - PubMed
-
- Asher JH, Jr., Harrison RW, Morell R, Carey ML, Friedman TB. Effects of Pax3 modifier genes on craniofacial morphology, pigmentation, and viability: A murine model of Waardenburg syndrome variation. Genomics. 1996;34:285–298. - PubMed
-
- Bruner JP, Tulipan N, Paschall RL, Boehm FH, Walsh WF, Silva SR, et al. Fetal surgery for myelomeningocele and the incidence of shunt- dependent hydrocephalus. JAMA. 1999;282:1819–1825. - PubMed
-
- Copp AJ, Greene NDE, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–793. - PubMed
-
- Correia-Pinto J, Reis JL, Hutchins GM, Baptista MJ, Estevao-Costa J, Flake AW, et al. In utero meconium exposure increases spinal cord necrosis in a rat model of myelomeningocele. J Pediatr Surg. 2002;37:488–492. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

