A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells
- PMID: 17465679
- PMCID: PMC1857715
- DOI: 10.1371/journal.ppat.0030060
A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells
Abstract
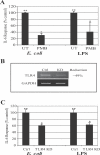
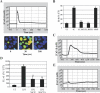
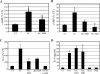
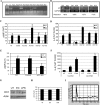
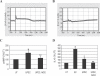
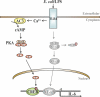
The vigorous cytokine response of immune cells to Gram-negative bacteria is primarily mediated by a recognition molecule, Toll-like receptor 4 (TLR4), which recognizes lipopolysaccharide (LPS) and initiates a series of intracellular NF-kappaB-associated signaling events. Recently, bladder epithelial cells (BECs) were reported to express TLR4 and to evoke a vigorous cytokine response upon exposure to LPS. We examined intracellular signaling events in human BECs leading to the production of IL-6, a major urinary cytokine, following activation by Escherichia coli and isolated LPS. We observed that in addition to the classical NF-kappaB-associated pathway, TLR4 triggers a distinct and more rapid signaling response involving, sequentially, Ca(2+), adenylyl cyclase 3-generated cAMP, and a transcriptional factor, cAMP response element-binding protein. This capacity of BECs to mobilize secondary messengers and evoke a more rapid IL-6 response might be critical in their role as first responders to microbial challenge in the urinary tract.
Conflict of interest statement
Figures


References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. - PubMed
-
- Haraoka M, Hang L, Frendeus B, Godaly G, Burdick M, et al. Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis. 1999;180:1220–1229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

