PICK1 interacts with alpha7 neuronal nicotinic acetylcholine receptors and controls their clustering
- PMID: 17467288
- PMCID: PMC3310904
- DOI: 10.1016/j.mcn.2007.03.009
PICK1 interacts with alpha7 neuronal nicotinic acetylcholine receptors and controls their clustering
Abstract
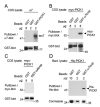
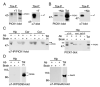
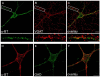
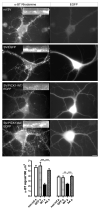
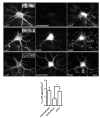
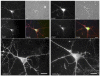
Central to synaptic function are protein scaffolds associated with neurotransmitter receptors. Alpha7 neuronal nicotinic acetylcholine receptors (nAChRs) modulate network activity, neuronal survival and cognitive processes in the CNS, but protein scaffolds that interact with these receptors are unknown. Here we show that the PDZ-domain containing protein PICK1 binds to alpha7 nAChRs and plays a role in their clustering. PICK1 interacted with the alpha7 cytoplasmic loop in yeast in a PDZ-dependent way, and the interaction was confirmed in recombinant pull-down experiments and by co-precipitation of native proteins. Some alpha7 and PICK1 clusters were adjacent at the surface of SH-SY5Y cells and GABAergic interneurons in hippocampal cultures. Expression of PICK1 caused decreased alpha7 clustering on the surface of the interneurons in a PDZ-dependent way. These data show that PICK1 negatively regulates surface clustering of alpha7 nAChRs on hippocampal interneurons, which may be important in inhibitory functions of alpha7 in the hippocampus.
Figures



References
-
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J. Pharmacol. Exp. Ther. 1997;283:1396–1411. - PubMed
-
- Alkondon M, Pereira EF, Albuquerque EX. Alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. - PubMed
-
- Archibald K, Perry MJ, Molnar E, Henley JM. Surface expression and metabolic half-life of AMPA receptors in cultured rat cerebellar granule cells. Neuropharmacology. 1998;37:1345–1353. - PubMed
-
- Ben-Ami HC, Yassin L, Farah H, Michaeli A, Eshel M, Treinin M. RIC-3 affects properties and quantity of nicotinic acetylcholine receptors via a mechanism that does not require the coiled-coil domains. J. Biol. Chem. 2005;280:28053–28060. - PubMed
-
- Berg DK, Conroy WG. Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. J. Neurobiol. 2002;53:512–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

