Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3)
- PMID: 17467345
- PMCID: PMC2099696
- DOI: 10.1016/j.jcf.2007.03.003
Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3)
Abstract
Background: Antimicrobial peptides (AMPs) such as cathelicidins contribute to initial defense of the airway against inhaled pathogens. Recent studies have shown that the hormonally active form of vitamin D(3), 1,25-dihydroxyvitamin D(3) (1,25(OH)(2)D(3)) up-regulates AMP gene expression in several established cell lines. Furthermore, serum levels of vitamin D are often deficient in cystic fibrosis (CF) patients.
Methods: We investigated the effect of 1,25(OH)(2)D(3) on AMP mRNA levels in primary cultures of normal human bronchial epithelial (NHBE) cells by real-time PCR, and protein levels by Western blot. Antimicrobial activity of airway surface fluid from these cells was measured by in vitro assay against laboratory strains of bacteria.
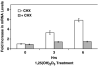
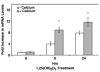
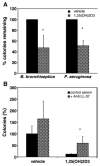
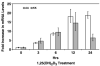
Results: Treatment of NHBE cells with 1,25(OH)(2)D(3) (10(-8)M), resulted in a 10-fold up-regulation of cathelicidin mRNA levels after 12 h, which was augmented 2-fold with co-incubation of 1 mM Calcium. Moreover, 1,25(OH)(2)D(3) induced antimicrobial activity against the airway pathogens Bordetella bronchiseptica and Pseudomonas aeruginosa. 1,25(OH)(2)D(3) induced cathelicidin mRNA expression equally in both normal and CF bronchial epithelial cells.
Conclusions: Elucidation of the effect of 1,25(OH)(2)D(3) on cathelicidin expression in NHBE cells and CF bronchial epithelial cells will aid in the development of novel therapeutic agents for treatment of airway infections in CF.
Figures

Similar articles
-
Induction of triggering receptor expressed on myeloid cells (TREM-1) in airway epithelial cells by 1,25(OH)₂ vitamin D₃.Innate Immun. 2012 Apr;18(2):250-7. doi: 10.1177/1753425911399796. Epub 2011 Jun 20. Innate Immun. 2012. PMID: 21690199 Free PMC article.
-
Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro.Antiviral Res. 2017 Jan;137:93-101. doi: 10.1016/j.antiviral.2016.11.004. Epub 2016 Nov 10. Antiviral Res. 2017. PMID: 27838350
-
Vitamin D receptor agonists inhibit pro-inflammatory cytokine production from the respiratory epithelium in cystic fibrosis.J Cyst Fibros. 2011 Dec;10(6):428-34. doi: 10.1016/j.jcf.2011.06.013. Epub 2011 Jul 23. J Cyst Fibros. 2011. PMID: 21784717
-
Species-specific regulation of innate immunity by vitamin D signaling.J Steroid Biochem Mol Biol. 2016 Nov;164:246-253. doi: 10.1016/j.jsbmb.2015.09.016. Epub 2015 Sep 11. J Steroid Biochem Mol Biol. 2016. PMID: 26369615 Review.
-
Antimicrobial peptides in the airway.Curr Top Microbiol Immunol. 2006;306:153-82. doi: 10.1007/3-540-29916-5_6. Curr Top Microbiol Immunol. 2006. PMID: 16909921 Review.
Cited by
-
Vitamin D and allergic airway disease shape the murine lung microbiome in a sex-specific manner.Respir Res. 2016 Sep 21;17(1):116. doi: 10.1186/s12931-016-0435-3. Respir Res. 2016. PMID: 27655266 Free PMC article.
-
Calcium and vitamin D in sarcoidosis: how to assess and manage.Semin Respir Crit Care Med. 2010 Aug;31(4):474-84. doi: 10.1055/s-0030-1262215. Epub 2010 Jul 27. Semin Respir Crit Care Med. 2010. PMID: 20665397 Free PMC article. Review.
-
Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey.Arch Intern Med. 2009 Feb 23;169(4):384-90. doi: 10.1001/archinternmed.2008.560. Arch Intern Med. 2009. PMID: 19237723 Free PMC article.
-
Vitamin D induction of the human antimicrobial Peptide cathelicidin in the urinary bladder.PLoS One. 2010 Dec 14;5(12):e15580. doi: 10.1371/journal.pone.0015580. PLoS One. 2010. PMID: 21179490 Free PMC article.
-
Vitamin D supplementation decreases Aspergillus fumigatus specific Th2 responses in CF patients with aspergillus sensitization: a phase one open-label study.Asthma Res Pract. 2015;1:3. doi: 10.1186/s40733-015-0003-5. Epub 2015 Jun 4. Asthma Res Pract. 2015. PMID: 27011794 Free PMC article.
References
-
- Laube DM, Yim S, Ryan LK, Kisich KO, Diamond G. Antimicrobial peptides in the airway. Curr Top Microbiol Immunol. 2006;306:153–82. - PubMed
-
- Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L799–805. - PubMed
-
- Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. - PubMed
-
- Ramanathan B, Davis EG, Ross CR, Blecha F. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 2002;4(3):361–72. - PubMed
-
- Zanetti M, Gennaro R, Scocchi M, Skerlavaj B. Structure and biology of cathelicidins. Adv Exp Med Biol. 2000;479:203–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

