Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia
- PMID: 17468117
- PMCID: PMC3023418
- DOI: 10.1093/brain/awm071
Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia
Abstract
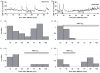
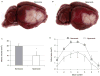
Normobaric hyperoxia is under investigation as a treatment for acute ischaemic stroke. In experimental models, normobaric hyperoxia reduces cerebral ischaemic injury and improves functional outcome. The mechanisms of neuroprotection are still debated because, (i) inhalation of 100% O2 does not significantly increase total blood O2 content; (ii) it is not known whether normobaric hyperoxia increases O2 delivery to the severely ischaemic cortex because of its short diffusion distance; and (iii) hyperoxia may reduce collateral cerebral blood flow (CBF) to ischaemic penumbra because it can cause vasoconstriction. We addressed these issues using real-time two-dimensional multispectral reflectance imaging and laser speckle flowmetry to simultaneously and non-invasively determine the impact of normobaric hyperoxia on CBF and oxygenation in ischaemic cortex. Ischaemia was induced by distal middle cerebral artery occlusion (dMCAO) in normoxic (30% inhaled O2, arterial pO2 134 +/- 9 mmHg), or hyperoxic mice (100% inhaled O2 starting 15 min after dMCAO, arterial pO2 312 +/- 10 mmHg). Post-ischaemic normobaric hyperoxia caused an immediate and progressive increase in oxyhaemoglobin (oxyHb) concentration, nearly doubling it in ischaemic core within 60 min. In addition, hyperoxia improved CBF so that the area of cortex with < or =20% residual CBF was decreased by 45% 60 min after dMCAO. Furthermore, hyperoxia reduced the frequency of peri-infarct depolarizations (PIDs) by more than 60%, and diminished their deleterious effects on CBF and metabolic load. Consistent with these findings, infarct size was reduced by 45% in the hyperoxia group 2 days after 75 min transient dMCAO. Our data show that normobaric hyperoxia increases tissue O2 delivery, and that novel mechanisms such as CBF augmentation, and suppression of PIDs may afford neuroprotection during hyperoxia.
Figures




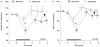
References
-
- Auer RN. Non-pharmacologic (physiologic) neuroprotection in the treatment of brain ischemia. Ann N Y Acad Sci. 2001;939:271–82. - PubMed
-
- Ayata C, Dunn AK, Gursoy OY, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24:744–55. - PubMed
-
- Bergo GW, Tyssebotn I. Effect of exposure to oxygen at 101 and 150 kPa on the cerebral circulation and oxygen supply in conscious rats. Eur J Appl Physiol Occup Physiol. 1995;71:475–84. - PubMed
-
- Branston NM, Symon L, Crockard HA. Recovery of the cortical evoked response following temporary middle cerebral artery occlusion in baboons: relation to local blood flow and PO2. Stroke. 1976;7:151–7. - PubMed
-
- Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas. 2001;22:R35–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01EB00790-01A2/EB/NIBIB NIH HHS/United States
- R01 EB000790/EB/NIBIB NIH HHS/United States
- R01-NS37074/NS/NINDS NIH HHS/United States
- P50 NS10828/NS/NINDS NIH HHS/United States
- R01 NS037074/NS/NINDS NIH HHS/United States
- P50 NS010828/NS/NINDS NIH HHS/United States
- R01 NS061505/NS/NINDS NIH HHS/United States
- K25 NS041291/NS/NINDS NIH HHS/United States
- P01 NS055104/NS/NINDS NIH HHS/United States
- P01 NS035611/NS/NINDS NIH HHS/United States
- K25NS041291/NS/NINDS NIH HHS/United States
- P01 NS35611/NS/NINDS NIH HHS/United States
- R01 NS056458/NS/NINDS NIH HHS/United States
- R01-NS56458/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

