The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants
- PMID: 17468213
- PMCID: PMC1914205
- DOI: 10.1104/pp.107.099648
The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants
Abstract
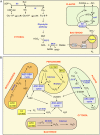
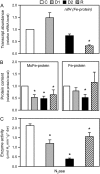
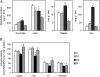
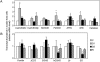
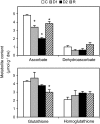
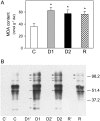
Alfalfa (Medicago sativa) plants were exposed to drought to examine the involvement of carbon metabolism and oxidative stress in the decline of nitrogenase (N(2)ase) activity. Exposure of plants to a moderate drought (leaf water potential of -1.3 MPa) had no effect on sucrose (Suc) synthase (SS) activity, but caused inhibition of N(2)ase activity (-43%), accumulation of succinate (+36%) and Suc (+58%), and up-regulation of genes encoding cytosolic CuZn-superoxide dismutase (SOD), plastid FeSOD, cytosolic glutathione reductase, and bacterial MnSOD and catalases B and C. Intensification of stress (-2.1 MPa) decreased N(2)ase (-82%) and SS (-30%) activities and increased malate (+40%), succinate (+68%), and Suc (+435%). There was also up-regulation (mRNA) of cytosolic ascorbate peroxidase and down-regulation (mRNA) of SS, homoglutathione synthetase, and bacterial catalase A. Drought stress did not affect nifH mRNA level or leghemoglobin expression, but decreased MoFe- and Fe-proteins. Rewatering of plants led to a partial recovery of the activity (75%) and proteins (>64%) of N(2)ase, a complete recovery of Suc, and a decrease of malate (-48%) relative to control. The increase in O(2) diffusion resistance, the decrease in N(2)ase-linked respiration and N(2)ase proteins, the accumulation of respiratory substrates and oxidized lipids and proteins, and the up-regulation of antioxidant genes reveal that bacteroids have their respiratory activity impaired and that oxidative stress occurs in nodules under drought conditions prior to any detectable effect on SS or leghemoglobin. We conclude that a limitation in metabolic capacity of bacteroids and oxidative damage of cellular components are contributing factors to the inhibition of N(2)ase activity in alfalfa nodules.
Figures

Similar articles
-
Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.).J Exp Bot. 2011 Jan;62(1):111-23. doi: 10.1093/jxb/erq249. Epub 2010 Aug 25. J Exp Bot. 2011. PMID: 20797998 Free PMC article.
-
Alfalfa nodules elicited by a flavodoxin-overexpressing Ensifer meliloti strain display nitrogen-fixing activity with enhanced tolerance to salinity stress.Planta. 2012 Dec;236(6):1687-700. doi: 10.1007/s00425-012-1725-8. Epub 2012 Aug 4. Planta. 2012. PMID: 22864594
-
Mechanisms of physiological adjustment of N2 fixation in Cicer arietinum L. (chickpea) during early stages of water deficit: single or multi-factor controls.Plant J. 2014 Sep;79(6):964-80. doi: 10.1111/tpj.12599. Epub 2014 Jul 28. Plant J. 2014. PMID: 24947137
-
Effects of engineered Sinorhizobium meliloti on cytokinin synthesis and tolerance of alfalfa to extreme drought stress.Appl Environ Microbiol. 2012 Nov;78(22):8056-61. doi: 10.1128/AEM.01276-12. Epub 2012 Sep 7. Appl Environ Microbiol. 2012. PMID: 22961897 Free PMC article.
-
Nodule-enhanced expression of a sucrose phosphate synthase gene member (MsSPSA) has a role in carbon and nitrogen metabolism in the nodules of alfalfa (Medicago sativa L.).Planta. 2010 Jan;231(2):233-44. doi: 10.1007/s00425-009-1043-y. Epub 2009 Nov 8. Planta. 2010. PMID: 19898977 Free PMC article.
Cited by
-
Reduced carbon availability to bacteroids and elevated ureides in nodules, but not in shoots, are involved in the nitrogen fixation response to early drought in soybean.Plant Physiol. 2007 Oct;145(2):539-46. doi: 10.1104/pp.107.102491. Epub 2007 Aug 24. Plant Physiol. 2007. PMID: 17720761 Free PMC article.
-
Control of the rhizobium-legume symbiosis by the plant nitrogen demand is tightly integrated at the whole plant level and requires inter-organ systemic signaling.Front Plant Sci. 2023 Mar 9;14:1114840. doi: 10.3389/fpls.2023.1114840. eCollection 2023. Front Plant Sci. 2023. PMID: 36968361 Free PMC article. Review.
-
Physiological, Hormonal and Metabolic Responses of two Alfalfa Cultivars with Contrasting Responses to Drought.Int J Mol Sci. 2019 Oct 15;20(20):5099. doi: 10.3390/ijms20205099. Int J Mol Sci. 2019. PMID: 31618819 Free PMC article.
-
Pea Efficiency of Post-drought Recovery Relies on the Strategy to Fine-Tune Nitrogen Nutrition.Front Plant Sci. 2020 Feb 27;11:204. doi: 10.3389/fpls.2020.00204. eCollection 2020. Front Plant Sci. 2020. PMID: 32174946 Free PMC article.
-
MtCAS31 Aids Symbiotic Nitrogen Fixation by Protecting the Leghemoglobin MtLb120-1 Under Drought Stress in Medicago truncatula.Front Plant Sci. 2018 May 14;9:633. doi: 10.3389/fpls.2018.00633. eCollection 2018. Front Plant Sci. 2018. PMID: 29868087 Free PMC article.
References
-
- Aebi H (1984) Catalase in vitro. Methods Enzymol 105 121–126 - PubMed
-
- Amako K, Chen GX, Asada K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35 497–504
-
- Appleby CA, Bergersen FJ (1980) Preparation and experimental use of leghemoglobin. In FJ Bergersen, ed, Methods for Evaluating Biological Nitrogen Fixation. John Wiley, Chichester, UK, pp 315–335
-
- Arrese-Igor C, González EM, Gordon AJ, Minchin FR, Gálvez L, Royuela M, Cabrerizo P, Aparicio-Tejo PM (1999) Sucrose synthase and nodule nitrogen fixation under drought and other environmental stresses. Symbiosis 27 189–212
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

