Clocking out: modeling phage-induced lysis of Escherichia coli
- PMID: 17468251
- PMCID: PMC1913442
- DOI: 10.1128/JB.00392-07
Clocking out: modeling phage-induced lysis of Escherichia coli
Erratum in
- J Bacteriol. 2007 Sep;189(17):6506
Abstract
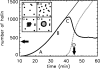
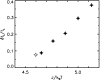
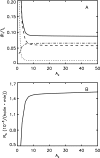
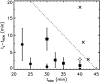
Phage lambda lyses the host Escherichia coli at a precisely scheduled time after induction. Lysis timing is determined by the action of phage holins, which are small proteins that induce hole formation in the bacterium's cytoplasmic membrane. We present a two-stage nucleation model of lysis timing, with the nucleation of condensed holin rafts on the inner membrane followed by the nucleation of a hole within those rafts. The nucleation of holin rafts accounts for most of the delay of lysis after induction. Our simulations of this model recover the accurate lysis timing seen experimentally and show that the timing accuracy is optimal. An enhanced holin-holin interaction is needed in our model to recover experimental lysis delays after the application of membrane poison, and such early triggering of lysis is possible only after the inner membrane is supersaturated with holin. Antiholin reduces the delay between membrane depolarization and lysis and leads to an earlier time after which triggered lysis is possible.
Figures

References
-
- Avron, J. E., H. van Beijeren, L. S. Schulman, and R. K. P. Zia. 1982. Roughening transition, surface tension and equilibrium droplet shapes in a two-dimensional Ising system. J. Phys. A 15:L81-L86.
-
- Bowie, J. U. 1997. Helix packing in membrane proteins. J. Mol. Bacteriol. 272:780-789. - PubMed
-
- Bray, A. J. 2002. Theory of phase-ordering kinetics. Adv. Phys. 51:481-587.
-
- Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

