Signal transduction and information processing in mammalian taste buds
- PMID: 17468883
- PMCID: PMC3723147
- DOI: 10.1007/s00424-007-0247-x
Signal transduction and information processing in mammalian taste buds
Abstract
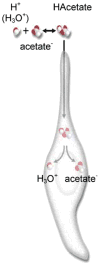
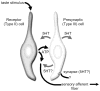
The molecular machinery for chemosensory transduction in taste buds has received considerable attention within the last decade. Consequently, we now know a great deal about sweet, bitter, and umami taste mechanisms and are gaining ground rapidly on salty and sour transduction. Sweet, bitter, and umami tastes are transduced by G-protein-coupled receptors. Salty taste may be transduced by epithelial Na channels similar to those found in renal tissues. Sour transduction appears to be initiated by intracellular acidification acting on acid-sensitive membrane proteins. Once a taste signal is generated in a taste cell, the subsequent steps involve secretion of neurotransmitters, including ATP and serotonin. It is now recognized that the cells responding to sweet, bitter, and umami taste stimuli do not possess synapses and instead secrete the neurotransmitter ATP via a novel mechanism not involving conventional vesicular exocytosis. ATP is believed to excite primary sensory afferent fibers that convey gustatory signals to the brain. In contrast, taste cells that do have synapses release serotonin in response to gustatory stimulation. The postsynaptic targets of serotonin have not yet been identified. Finally, ATP secreted from receptor cells also acts on neighboring taste cells to stimulate their release of serotonin. This suggests that there is important information processing and signal coding taking place in the mammalian taste bud after gustatory stimulation.
Figures

References
-
- Simon SA, de Araujo I, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci. 2006;7:890–901. - PubMed
-
- Meyerhof W. Elucidation of mammalian bitter taste. Rev Physiol Biochem Pharmacol. 2005;154:37–72. - PubMed
-
- Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev. 2005;4:143–191. - PubMed
-
- Breslin PA, Huang L. Human taste: peripheral anatomy, taste transduction, and coding. Adv Oto-Rhino-Laryngol. 2006;63:152–190. - PubMed
-
- Gilbertson TA. Gustatory mechanisms for the detection of fat. Curr Opin Neurobiol. 1998;8:447–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

