Stabilized phosphatidylinositol-5-phosphate analogues as ligands for the nuclear protein ING2: chemistry, biology, and molecular modeling
- PMID: 17469822
- PMCID: PMC2553394
- DOI: 10.1021/ja070195b
Stabilized phosphatidylinositol-5-phosphate analogues as ligands for the nuclear protein ING2: chemistry, biology, and molecular modeling
Abstract
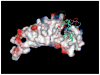
The interaction of PtdIns(5)P with the tumor suppressor protein ING2 has been implicated in the regulation of chromatin modification. To enhance the stability of PtdIns(5)P for studies of the biological role in vivo, two phosphatase-resistant moieties were used to replace the labile 5-phosphate. The total asymmetric synthesis of the 5-methylenephosphonate (MP) and 5-phosphothionate (PT) analogues of PtdIns(5)P is described herein, and the resulting metabolically stabilized lipid analogues were evaluated in three ways. First, liposomes containing either the dioleoyl MP or PT analogues bound to recombinant ING2 similar to liposomes containing dipalmitoyl PtdIns(5)P, indicating that the replacement of the hydrolyzable 5-phosphate group does not compromise the binding. Second, the dioleoyl MP and PT PtdIns(5)P analogues were equivalent to dipalmitoyl PtdIns(5)P in augmenting cell death induced by a DNA double-strand break in HT1080 cells. Finally, molecular modeling and docking of the MP or PT analogues to the C-terminus PtdInsP-binding region of ING2 (consisting of a PHD finger and a polybasic region) revealed a number of complementary surface and electrostatic contacts between the lipids and ING2.
Figures
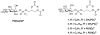






Similar articles
-
Nuclear phosphatidylinositol-5-phosphate regulates ING2 stability at discrete chromatin targets in response to DNA damage.Sci Rep. 2013;3:2137. doi: 10.1038/srep02137. Sci Rep. 2013. PMID: 23823870 Free PMC article.
-
The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor.Cell. 2003 Jul 11;114(1):99-111. doi: 10.1016/s0092-8674(03)00480-x. Cell. 2003. PMID: 12859901
-
Leucine zipper-like domain is required for tumor suppressor ING2-mediated nucleotide excision repair and apoptosis.FEBS Lett. 2006 Jul 10;580(16):3787-93. doi: 10.1016/j.febslet.2006.05.065. Epub 2006 Jun 9. FEBS Lett. 2006. PMID: 16782091
-
ING1 and ING2: multifaceted tumor suppressor genes.Cell Mol Life Sci. 2013 Oct;70(20):3753-72. doi: 10.1007/s00018-013-1270-z. Epub 2013 Feb 15. Cell Mol Life Sci. 2013. PMID: 23412501 Free PMC article. Review.
-
The return of the INGs, histone mark sensors and phospholipid signaling effectors.Curr Drug Targets. 2009 May;10(5):418-31. doi: 10.2174/138945009788185112. Curr Drug Targets. 2009. PMID: 19442114 Review.
Cited by
-
Conditional peripheral membrane proteins: facing up to limited specificity.Structure. 2012 Jan 11;20(1):15-27. doi: 10.1016/j.str.2011.11.012. Epub 2011 Dec 21. Structure. 2012. PMID: 22193136 Free PMC article. Review.
-
Metabolically stabilized derivatives of phosphatidylinositol 4-phosphate: synthesis and applications.Chem Biol. 2011 Oct 28;18(10):1312-9. doi: 10.1016/j.chembiol.2011.07.022. Chem Biol. 2011. PMID: 22035800 Free PMC article.
-
ING2, a tumor associated gene, enhances PAI‑1 and HSPA1A expression with HDAC1 and mSin3A through the PHD domain and C‑terminal.Mol Med Rep. 2017 Nov;16(5):7367-7374. doi: 10.3892/mmr.2017.7553. Epub 2017 Sep 20. Mol Med Rep. 2017. PMID: 28944862 Free PMC article.
-
A scalable synthesis of the IP7 isomer, 5-PP-Ins(1,2,3,4,6)P5.Org Lett. 2009 Apr 2;11(7):1551-4. doi: 10.1021/ol900149x. Org Lett. 2009. PMID: 19253999 Free PMC article.
-
Nuclear phosphatidylinositol-5-phosphate regulates ING2 stability at discrete chromatin targets in response to DNA damage.Sci Rep. 2013;3:2137. doi: 10.1038/srep02137. Sci Rep. 2013. PMID: 23823870 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

