Clinical and neurobiological aspects of narcolepsy
- PMID: 17470414
- PMCID: PMC1978248
- DOI: 10.1016/j.sleep.2007.03.008
Clinical and neurobiological aspects of narcolepsy
Abstract
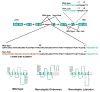
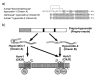
Narcolepsy is characterized by excessive daytime sleepiness (EDS), cataplexy and/or other dissociated manifestations of rapid eye movement (REM) sleep (hypnagogic hallucinations and sleep paralysis). Narcolepsy is currently treated with amphetamine-like central nervous system (CNS) stimulants (for EDS) and antidepressants (for cataplexy). Some other classes of compounds such as modafinil (a non-amphetamine wake-promoting compound for EDS) and gamma-hydroxybutyrate (GHB, a short-acting sedative for EDS/fragmented nighttime sleep and cataplexy) given at night are also employed. The major pathophysiology of human narcolepsy has been recently elucidated based on the discovery of narcolepsy genes in animals. Using forward (i.e., positional cloning in canine narcolepsy) and reverse (i.e., mouse gene knockout) genetics, the genes involved in the pathogenesis of narcolepsy (hypocretin/orexin ligand and its receptor) in animals have been identified. Hypocretins/orexins are novel hypothalamic neuropeptides also involved in various hypothalamic functions such as energy homeostasis and neuroendocrine functions. Mutations in hypocretin-related genes are rare in humans, but hypocretin-ligand deficiency is found in many narcolepsy-cataplexy cases. In this review, the clinical, pathophysiological and pharmacological aspects of narcolepsy are discussed.
Figures




References
-
- Gélineau JBE. De la narcolepsie. Gazette des hôpitaux. 1880;53:626–628.
-
- Billiard M, Besset A, Cadilhac J. The clinical and polygraphic development of narcolepsy. In: Guilleminault C, Lugaresi E, editors. Sleep/wake disorders: natural history, epidemiology and longterm evolution. New York: Raven Press; 1983. pp. 171–185.
-
- Sonka K, Tafti M, Billiard M. Narcolepsy and aging. In: Smiru S, Frauceschi M, Ferini-Strambi L, editors. Sleep and aging. Milano: Masson; 1991. pp. 181–186.
-
- Guilleminault C. Narcolepsy Syndrome. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 2. Philadelphia: W. B. Saunders; 1994. pp. 549–561.
-
- Hublin C, Kaprio J, Partinene M, Koskenvuo M, Heikkila K, Koskimies S, et al. The prevalence of narcolepsy: an epidemiological study of the Finnish twin cohort. Ann Neurol. 1994;35:709–716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

