Development of fatal colitis in FVB mice infected with Citrobacter rodentium
- PMID: 17470543
- PMCID: PMC1932959
- DOI: 10.1128/IAI.01810-06
Development of fatal colitis in FVB mice infected with Citrobacter rodentium
Abstract
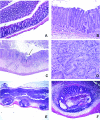
Citrobacter rodentium is the causative agent of transmissible murine colonic hyperplasia. The disease is characterized by severe but temporary epithelial hyperplasia with limited inflammation in the descending colon of adult mice on a variety of genetic backgrounds. The natural history of infection with this murine pathogen has been characterized in outbred Swiss Webster (SW) mice but not in the cognate inbred FVB strain. In contrast to subclinical infection in SW mice, 12-week-old FVB mice developed overt disease with significant weight loss and mortality beginning by 9 days postinoculation (dpi). By 21 dpi, more than 75% of infected FVB mice died or had to be euthanized, whereas no mortality developed in SW mice. Mortality in FVB mice was fully prevented by fluid therapy. Fecal shedding of bacteria was similar in both groups through 9 dpi; however, a slight but significant delay in bacterial clearance was observed in FVB mice by 12 to 18 dpi. SW mice developed hyperplasia with minimal inflammation in the descending colon. FVB mice developed epithelial cell hyperproliferation, severe inflammation with erosions and ulcers, and epithelial atypia by 6 dpi in the descending colon. In the majority of surviving FVB mice, colonic lesions, including epithelial atypia, were reversible, although a small percentage (5 to 7%) exhibited chronic colitis through 7 months postinoculation. The existence of susceptible and resistant lines of mice with similar genetic backgrounds will facilitate the identification of host factors responsible for the outcome of infection and may lead to the development of novel strategies for preventing and treating infectious colitis.
Figures




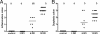


References
-
- Barthold, S. W. 1979. Autoradiographic cytokinetics of colonic mucosal hyperplasia in mice. Cancer Res. 39:24-29. - PubMed
-
- Barthold, S. W. 1980. The microbiology of transmissible murine colonic hyperplasia. Lab. Anim. Sci. 30:167-173. - PubMed
-
- Barthold, S. W., and A. M. Jonas. 1977. Morphogenesis of early 1,2-dimethylhydrazine-induced lesions and latent period reduction of colon carcinogenesis in mice by a variant of Citrobacter freundii. Cancer Res. 37:4352-4360. - PubMed
-
- Barthold, S. W., and D. Beck. 1980. Modification of early dimethylhydrazine carcinogenesis by colonic mucosal hyperplasia. Cancer Res. 40:4451-4455. - PubMed
-
- Barthold, S. W., G. L. Coleman, P. N. Bhatt, G. W. Osbaldiston, and A. M. Jonas. 1976. The etiology of transmissible murine colonic hyperplasia. Lab. Anim. Sci. 26:889-894. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

