The Neisseria meningitidis capsule is important for intracellular survival in human cells
- PMID: 17470547
- PMCID: PMC1932921
- DOI: 10.1128/IAI.01945-06
The Neisseria meningitidis capsule is important for intracellular survival in human cells
Abstract
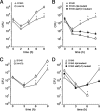
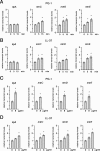
While much data exist in the literature about how Neisseria meningitidis adheres to and invades human cells, its behavior inside the host cell is largely unknown. One of the essential meningococcal attributes for pathogenesis is the polysaccharide capsule, which has been shown to be important for bacterial survival in extracellular fluids. To investigate the role of the meningococcal capsule in intracellular survival, we used B1940, a serogroup B strain, and its isogenic derivatives, which lack either the capsule or both the capsule and the lipooligosaccharide outer core, to infect human phagocytic and nonphagocytic cells and monitor invasion and intracellular growth. Our data indicate that the capsule, which negatively affects bacterial adhesion and, consequently, entry, is, in contrast, fundamental for the intracellular survival of this microorganism. The results of in vitro assays suggest that an increased resistance to cationic antimicrobial peptides (CAMPs), important components of the host innate defense system against microbial infections, is a possible mechanism by which the capsule protects the meningococci in the intracellular environment. Indeed, unencapsulated bacteria were more susceptible than encapsulated bacteria to defensins, cathelicidins, protegrins, and polymyxin B, which has long been used as a model compound to define the mechanism of action of CAMPs. We also demonstrate that both the capsular genes (siaD and lipA) and those encoding an efflux pump involved in resistance to CAMPs (mtrCDE) were up-regulated during the intracellular phase of the infectious cycle.
Figures




References
-
- Bucci, C., A. Lavitola, P. Salvatore, L. Del Giudice, D. Massardo, C. Bruni, and P. Alifano. 1999. Hypermutation in pathogenic bacteria: frequent phase variation in meningococci is a phenotypic trait of a specialized mutator biotype. Mol. Cell 3:435-445. - PubMed
-
- Deghmane, A., D. Giorgini, M. Larribe, J. Alonso, and M. Taha. 2002. Down-regulation of pili and capsule of Neisseria meningitidis upon contact with epithelial cells is mediated by CrgA regulatory protein. Mol. Microbiol. 43:1555-1564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

