Vascular endothelial growth factor can signal through platelet-derived growth factor receptors
- PMID: 17470632
- PMCID: PMC2064818
- DOI: 10.1083/jcb.200608093
Vascular endothelial growth factor can signal through platelet-derived growth factor receptors
Abstract
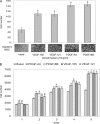
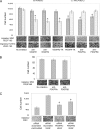
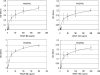
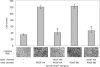
Vascular endothelial growth factor (VEGF-A) is a crucial stimulator of vascular cell migration and proliferation. Using bone marrow-derived human adult mesenchymal stem cells (MSCs) that did not express VEGF receptors, we provide evidence that VEGF-A can stimulate platelet-derived growth factor receptors (PDGFRs), thereby regulating MSC migration and proliferation. VEGF-A binds to both PDGFRalpha and PDGFRbeta and induces tyrosine phosphorylation that, when inhibited, results in attenuation of VEGF-A-induced MSC migration and proliferation. This mechanism was also shown to mediate human dermal fibroblast (HDF) migration. VEGF-A/PDGFR signaling has the potential to regulate vascular cell recruitment and proliferation during tissue regeneration and disease.
Figures





References
-
- Abedin, M., Y. Tintut, and L. Demer. 2004. Mesenchymal stem cells and the artery wall. Circ. Res. 95:671–676. - PubMed
-
- Aghi, M., and E.A. Chiocca. 2005. Contribution of bone marrow-derived cells to blood vessels in ischemic tissues and tumors. Mol. Ther. 12:994–1005. - PubMed
-
- Annabi, B., Y.-T. Lee, S. Turcotte, E. Naud, R.R. Desrosiers, M. Champagne, N. Eliopoulos, J. Galipeau, and R. Beliveau. 2003. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 21:337–347. - PubMed
-
- Ball, S.G., A.C. Shuttleworth, and C.M. Kielty. 2004. Direct cell contact influences bone marrow mesenchymal stem cell fate. Int. J. Biochem. Cell Biol. 36:714–727. - PubMed
-
- Ball, S.G., A.C. Shuttleworth, and C.M. Kielty. 2007. Platelet-derived growth factor receptor-alpha is a key determinant of smooth muscle alpha-actin filaments in bone marrow-derived mesenchymal stem cells. Int. J. Biochem. Cell Biol. 39:379–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

