Selective inhibition of porcine endogenous retrovirus replication in human cells by acyclic nucleoside phosphonates
- PMID: 17470654
- PMCID: PMC1913248
- DOI: 10.1128/AAC.00212-07
Selective inhibition of porcine endogenous retrovirus replication in human cells by acyclic nucleoside phosphonates
Abstract
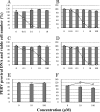
Several anti-human immunodeficiency virus type 1 reverse transcriptase inhibitors were evaluated for their antiviral activities against porcine endogenous retrovirus in human cells. Among the test compounds, zidovudine was found to be the most active. The order of potency was zidovudine > phosphonylmethoxyethoxydiaminopyrimidine = phosphonylmethoxypropyldiaminopurine > tenofovir > or = adefovir > stavudine.
Figures

Similar articles
-
Susceptibility of the porcine endogenous retrovirus to reverse transcriptase and protease inhibitors.J Virol. 2001 Jan;75(2):1048-53. doi: 10.1128/JVI.75.2.1048-1053.2001. J Virol. 2001. PMID: 11134319 Free PMC article.
-
Susceptibility of human T cell leukemia virus type I to nucleoside reverse transcriptase inhibitors.J Infect Dis. 2003 Aug 1;188(3):424-7. doi: 10.1086/376531. Epub 2003 Jul 10. J Infect Dis. 2003. PMID: 12870124
-
In vitro combination studies of tenofovir and other nucleoside analogues with ribavirin against HIV-1.Antivir Ther. 2005;10(2):343-8. Antivir Ther. 2005. PMID: 15865229
-
Mechanisms of HIV-1 nucleoside reverse transcriptase inhibitor resistance: is it all figured out?Curr Opin Investig Drugs. 2001 Mar;2(3):335-9. Curr Opin Investig Drugs. 2001. PMID: 11575701 Review.
-
Unachieved antiviral strategies with acyclic nucleoside phosphonates: Dedicated to the memory of dr. Salvatore "Sam" Joseph Enna.Biochem Pharmacol. 2024 Oct;228:116448. doi: 10.1016/j.bcp.2024.116448. Epub 2024 Jul 21. Biochem Pharmacol. 2024. PMID: 39043335 Review.
Cited by
-
Infection in xenotransplantation: opportunities and challenges.Curr Opin Organ Transplant. 2019 Oct;24(5):527-534. doi: 10.1097/MOT.0000000000000682. Curr Opin Organ Transplant. 2019. PMID: 31385886 Free PMC article. Review.
-
Surveillance and prevention of infection in clinical xenotransplantation.Clin Microbiol Rev. 2025 Mar 13;38(1):e0015023. doi: 10.1128/cmr.00150-23. Epub 2025 Jan 31. Clin Microbiol Rev. 2025. PMID: 39887237 Review.
-
Can Antiretroviral Drugs Be Used to Treat Porcine Endogenous Retrovirus (PERV) Infection after Xenotransplantation?Viruses. 2017 Aug 8;9(8):213. doi: 10.3390/v9080213. Viruses. 2017. PMID: 28786944 Free PMC article. Review.
-
Susceptibility of porcine endogenous retrovirus to anti-retroviral inhibitors.Xenotransplantation. 2016 Mar;23(2):151-8. doi: 10.1111/xen.12230. Epub 2016 Mar 29. Xenotransplantation. 2016. PMID: 27028725 Free PMC article.
References
-
- Balzarini, J., P. Herdewijn, and E. De Clercq. 1989. Differential patterns of intracellular metabolism of 2′,3′-didehydro-2′,3′-dideoxythymidine and 3′-azido-2′,3′-dideoxythymidine, two potent anti-human immunodeficiency virus compounds. J. Biol. Chem. 2646127-6133. - PubMed
-
- Cooper, D. K., and A. M. Keogh. 2001. The potential role of xenotransplantation in treating endstage cardiac disease: a summary of the report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation. Curr. Opin. Cardiol. 16105-109. - PubMed
-
- De Clercq, E. 2007. The acyclic nucleoside phosphonates from inception to clinical use: historical perspective. Antiviral Res. 751-13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information

