Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel
- PMID: 17470814
- PMCID: PMC1876545
- DOI: 10.1073/pnas.0702638104
Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel
Abstract
It is now well established that the voltage-sensing S4 segment in voltage-dependent ion channels undergoes a conformational change in response to varying membrane potential. However, the magnitude of the movement of S4 relative to the membrane and the rest of the protein remains controversial. Here, by using histidine scanning mutagenesis in the Shaker K channel, we identified mutants I241H (S1 segment) and I287H (S2 segment) that generate inward currents at hyperpolarized potentials, suggesting that these residues are part of a hydrophobic plug that separates the water-accessible crevices. Additional experiments with substituted cysteine residues showed that, at hyperpolarized potentials, both I241C and I287C can spontaneously form disulphide and metal bridges with R362C, the position of the first charge-carrying residue in S4. These results constrain unambiguously the closed-state positions of the S4 segment with respect to the S1 and S2 segments, which are known to undergo little or no movement during gating. To satisfy these constraints, the S4 segment must undergo an axial rotation of approximately 180 degrees and a transmembrane (vertical) movement of approximately 6.5 A at the level of R362 in going from the open to the closed state of the channel, moving the gating charge across a focused electric field.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

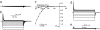
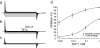
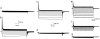
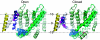
References
-
- Aggarwal SK, MacKinnon R. Neuron. 1996;16:1169–1177. - PubMed
-
- Seoh S-A, Sigg D, Papazian DM, Bezanilla F. Neuron. 1996;16:1159–1167. - PubMed
-
- Cha A, Snyder G, Selvin PR, Bezanilla F. Nature. 1999;402:809–813. - PubMed
-
- Chanda B, Asamoah OK, Blunck R, Roux B, Bezanilla F. Nature. 2005;436:852–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

