Hypoxia and the antipredator behaviours of fishes
- PMID: 17472921
- PMCID: PMC2442856
- DOI: 10.1098/rstb.2007.2103
Hypoxia and the antipredator behaviours of fishes
Abstract
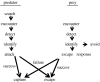
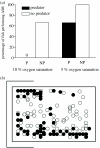
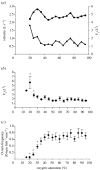
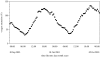
Hypoxia is a phenomenon occurring in marine coastal areas with increasing frequency. While hypoxia has been documented to affect fish activity and metabolism, recent evidence shows that hypoxia can also have a detrimental effect on various antipredator behaviours. Here, we review such evidence with a focus on the effect of hypoxia on fish escape responses, its modulation by aquatic surface respiration (ASR) and schooling behaviour. The main effect of hypoxia on escape behaviour was found in responsiveness and directionality. Locomotor performance in escapes was expected to be relatively independent of hypoxia, since escape responses are fuelled anaerobically. However, hypoxia decreased locomotor performance in some species (Mugilidae) although only in the absence of ASR in severe hypoxia. ASR allows fish to show higher escape performance than fish staying in the water column where hypoxia occurs. This situation provides a trade-off whereby fish may perform ASR in order to avoid the detrimental effects of hypoxia, although they would be subjected to higher exposure to aerial predation. As a result of this trade-off, fishes appear to minimize surfacing behaviour in the presence of aerial predators and to surface near shelters, where possible. For many fish species, schooling can be an effective antipredator behaviour. Severe hypoxia may lead to the disruption of the school unit. At moderate levels, hypoxia can increase school volume and can change the shuffling behaviour of individuals. By altering school structure and dynamics, hypoxia may affect the well functioning of schooling in terms of synchronization and execution of antipredator manoeuvres. School structure and volume appear to be the results of numerous trade-offs, where school shape may be dictated by the presence of predators, the need for energy saving via hydrodynamic advantages and oxygen level. The effects of hypoxia on aquatic organisms can be taxon specific. While hypoxia may not necessarily increase the vulnerability of fish subject to predation by other fish (since feeding in fish also decreases in hypoxia), predators from other taxa such as birds, jellyfish or aquatic mammals may take advantage of the detrimental effects of hypoxia on fish escape ability. Therefore, the effect of hypoxia on fish antipredator behaviours may have major consequences for the composition of aquatic communities.
Figures






References
-
- Abrahams M.V, Colgan P. Risk of predation, hydrodynamic efficiency and their influence on school structure. Environ. Biol. Fishes. 1985;13:195–202. doi:10.1007/BF00000931 - DOI
-
- Barber J, Walker P, Svensson P.A. Behavioural responses to simulated avian predation in female three spined sticklebacks: the effect of experimental Schistocephalus solidus infections. Behaviour. 2004;141:1425–1440. doi:10.1163/1568539042948231 - DOI
-
- Batty R.S, Domenici P. Predator–prey relationships in fish and other aquatic vertebrates: kinematics and behaviour. In: Domenici P, Blake R.W, editors. Biomechanics in animal behaviour. BIOS Scientific Publishers Ltd; Oxford, UK: 2000. pp. 237–257.
-
- Beamish F.W.H. Respiration of fish with special emphasis on standard oxygen consumption, III. Influence of oxygen. Can. J. Zool. 1964;42:355–366.
-
- Beamish F.W.H. In: Swimming capacity. Fish physiology. Hoar W.S, Randall D.J, editors. vol. 7. Academic Press, Inc; New York, NY: 1978. pp. 101–187.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
