Predator-prey interactions and changing environments: who benefits?
- PMID: 17472922
- PMCID: PMC2442855
- DOI: 10.1098/rstb.2007.2102
Predator-prey interactions and changing environments: who benefits?
Abstract
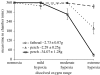
While aquatic environments have long been thought to be more moderate environments than their terrestrial cousins, environmental data demonstrate that for some systems this is not so. Numerous important environmental parameters can fluctuate dramatically, notably dissolved oxygen, turbidity and temperature. The roles of dissolved oxygen and turbidity on predator-prey interactions have been discussed in detail elsewhere within this issue and will be considered only briefly here. Here, we will focus primarily on the role of temperature and its potential impact upon predator-prey interactions. Two key properties are of particular note. For temperate aquatic ecosystems, all piscine and invertebrate piscivores and their prey are ectothermic. They will therefore be subject to energetic demands that are significantly affected by environmental temperature. Furthermore, the physical properties of water, particularly its high thermal conductivity, mean that thermal microenvironments will not exist so that fine-scale habitat movements will not be an option for dealing with changing water temperature in lentic environments. Unfortunately, there has been little experimental analysis of the role of temperature on such predator-prey interactions, so we will instead focus on theoretical work, indicating that potential implications associated with thermal change are unlikely to be straightforward and may present a greater threat to predators than to their prey. Specifically, we demonstrate that changes in the thermal environment can result in a net benefit to cold-adapted species through the mechanism of predator-prey interactions.
Figures
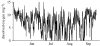
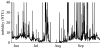




References
-
- Abrahams M.V. Risk of predation and its influence on the relative competitive abilities of two species of freshwater fishes. Can. J. Fish. Aquat. Sci. 1994;51:1629–1633.
-
- Abrahams M.V. The physiology of antipredator behaviour: what you do with what you've got. In: Sloman K.A, Wilson R.W, Balshine S, editors. Behaviour and physiology of fish; Vol. 24, Fish physiology. Academic Press; London, UK: 2005. pp. 79–108.
-
- Abrahams M.V, Dill L.M. A determination of the energetic equivalence of the risk of predation. Ecology. 1989;70:999–1007. doi:10.2307/1941368 - DOI
-
- Abrahams M.V, Kattenfeld M.G. The role of turbidity as a constraint on predator–prey interactions in aquatic environments. Behav. Ecol. Sociobiol. 1997;40:169–174. doi:10.1007/s002650050330 - DOI
-
- Abrahams, M. V. & Sloan, J. Submitted. Hypoxic environments and their impact upon habitat selection decisions involving the risk of predation.
MeSH terms
LinkOut - more resources
Full Text Sources
