Structure of the fungal beta-glucan-binding immune receptor dectin-1: implications for function
- PMID: 17473009
- PMCID: PMC2206667
- DOI: 10.1110/ps.072791207
Structure of the fungal beta-glucan-binding immune receptor dectin-1: implications for function
Abstract
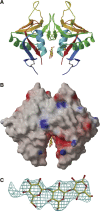
The murine molecule dectin-1 (known as the beta-glucan receptor in humans) is an immune cell surface receptor implicated in the immunological defense against fungal pathogens. Sequence analysis has indicated that the dectin-1 extracellular domain is a C-type lectin-like domain, and functional studies have established that it binds fungal beta-glucans. We report several dectin-1 crystal structures, including a high-resolution structure and a 2.8 angstroms resolution structure in which a short soaked natural beta-glucan is trapped in the crystal lattice. In vitro characterization of dectin-1 in the presence of its natural ligand indicates higher-order complex formation between dectin-1 and beta-glucans. These combined structural and biophysical data considerably extend the current knowledge of dectin-1 structure and function, and suggest potential mechanisms of defense against fungal pathogens.
Figures



Similar articles
-
Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching.J Pharmacol Exp Ther. 2008 Apr;325(1):115-23. doi: 10.1124/jpet.107.133124. Epub 2008 Jan 2. J Pharmacol Exp Ther. 2008. PMID: 18171906
-
Ligands for the beta-glucan receptor, Dectin-1, assigned using "designer" microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides.J Biol Chem. 2006 Mar 3;281(9):5771-9. doi: 10.1074/jbc.M511461200. Epub 2005 Dec 21. J Biol Chem. 2006. PMID: 16371356
-
Characterization of beta-glucan recognition site on C-type lectin, dectin 1.Infect Immun. 2004 Jul;72(7):4159-71. doi: 10.1128/IAI.72.7.4159-4171.2004. Infect Immun. 2004. PMID: 15213161 Free PMC article.
-
beta-Glucans and dectin-1.Ann N Y Acad Sci. 2008 Nov;1143:45-60. doi: 10.1196/annals.1443.019. Ann N Y Acad Sci. 2008. PMID: 19076344 Review.
-
3D Structural Insights into β-Glucans and Their Binding Proteins.Int J Mol Sci. 2021 Feb 4;22(4):1578. doi: 10.3390/ijms22041578. Int J Mol Sci. 2021. PMID: 33557270 Free PMC article. Review.
Cited by
-
Dectin-1 activation controls maturation of β-1,3-glucan-containing phagosomes.J Biol Chem. 2013 May 31;288(22):16043-54. doi: 10.1074/jbc.M113.473223. Epub 2013 Apr 22. J Biol Chem. 2013. PMID: 23609446 Free PMC article.
-
Structure of the human NK cell NKR-P1:LLT1 receptor:ligand complex reveals clustering in the immune synapse.Nat Commun. 2022 Aug 26;13(1):5022. doi: 10.1038/s41467-022-32577-6. Nat Commun. 2022. PMID: 36028489 Free PMC article.
-
Immunogenetics associated with severe coccidioidomycosis.JCI Insight. 2022 Nov 22;7(22):e159491. doi: 10.1172/jci.insight.159491. JCI Insight. 2022. PMID: 36166305 Free PMC article.
-
Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses.J Immunol. 2010 Sep 15;185(6):3504-13. doi: 10.4049/jimmunol.1000999. Epub 2010 Aug 20. J Immunol. 2010. PMID: 20729328 Free PMC article.
-
Identification and characterization of Cryptosporidium parvum Clec, a novel C-type lectin domain-containing mucin-like glycoprotein.Infect Immun. 2013 Sep;81(9):3356-65. doi: 10.1128/IAI.00436-13. Epub 2013 Jul 1. Infect Immun. 2013. PMID: 23817613 Free PMC article.
References
-
- Ariizumi K., Shen, G.L., Shikano, S., Xu, S., Ritter, R., Kumamoto, T., Edelbaum, D., Morita, A., Bergstresser, P.R., and Takashima, A. 2000. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 275: 20157–20167. - PubMed
-
- Bohn J.A. and BeMiller, J.N. 1995. (1–3)-β-D-glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 28: 3–14.
-
- Brown G.D. 2006. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6: 33–43. - PubMed
-
- Brown G.D. and Gordon, S. 2001. Immune recognition: A new receptor for β-glucans. Nature 413: 36–37. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

