Design and validation of an H5 TaqMan real-time one-step reverse transcription-PCR and confirmatory assays for diagnosis and verification of influenza A virus H5 infections in humans
- PMID: 17473050
- PMCID: PMC1865909
- DOI: 10.1128/JCM.02007-06
Design and validation of an H5 TaqMan real-time one-step reverse transcription-PCR and confirmatory assays for diagnosis and verification of influenza A virus H5 infections in humans
Abstract
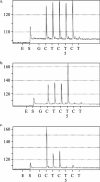
Increasing diversity among influenza H5N1 viruses has resulted in the need for sensitive and specific diagnostic assays, fully validated for the detection of H5 viruses belonging to all hemagglutinin (HA) clades, particularly the recently circulating H5N1 viruses of clade 2. In this report, the development and validation of a real-time, one-step TaqMan reverse transcription-PCR (RT-PCR) assay specific for the detection of influenza A H5 viruses from clades 1, 1', 2, and 3 is described. The real-time assay for H5 virus was shown to be highly sensitive, detecting H5 virus levels of <1 PFU from each of the HA clades. Specificity of the H5 RT-PCR for influenza A H5 viruses was demonstrated by using influenza A viruses of different subtypes, clinical samples containing influenza A viruses H1N1, H3N2, and H5N1, influenza B viruses, and other respiratory viruses. The usefulness of the inclusion of a distinguishable assay positive control and of confirmatory assays for the laboratory diagnosis and verification of H5 virus infections was demonstrated. A real-time RT-PCR pyrosequencing assay, a restriction enzyme digestion assay, and direct sequencing of the H5 real-time RT-PCR amplicon were validated for the confirmation of H5 detection by the diagnostic real-time assay. The H5 real-time assay was applied to diagnostic testing for suspected cases of influenza A virus H5 infection in the United Kingdom. Influenza A H5 viruses were not detected in the cases analyzed; however, influenza A H3N2 virus was detected in 57% of the suspected cases of H5. The H5 TaqMan real-time RT-PCR and confirmatory assays will be useful tools for the laboratory surveillance and rapid diagnosis of H5 infections in humans.
Figures

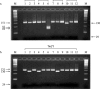
References
-
- Adelson, M. E., M. Feola, J. Trama, R. C. Tilton, and E. Mordechai. 2005. Simultaneous detection of herpes simplex virus types 1 and 2 by real-time PCR and pyrosequencing. J. Clin. Virol. 33:25-34. - PubMed
-
- Bernard, P. S., and C. T. Wittwer. 2002. Real-time PCR technology for cancer diagnostics. Clin. Chem. 48:1178-1185. - PubMed
-
- Borst, A., A. T. Box, and A. C. Fluit. 2004. False-positive results and contamination in nucleic acid amplification assays: suggestions for a prevent and destroy strategy. Eur. J. Clin. Microbiol. Infect. Dis. 23:289-299. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

