Second-line paclitaxel in non-small cell lung cancer initially treated with cisplatin: a study by the European Lung Cancer Working Party
- PMID: 17473825
- PMCID: PMC2359915
- DOI: 10.1038/sj.bjc.6603772
Second-line paclitaxel in non-small cell lung cancer initially treated with cisplatin: a study by the European Lung Cancer Working Party
Abstract
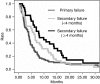
In the context of a phase III trial comparing in advanced non-small cell lung cancer (NSCLC) sequential to conventional administration of cisplatin-based chemotherapy and paclitaxel, we evaluated the activity of paclitaxel as second-line chemotherapy and investigated any relation of its efficacy with the type of failure after cisplatin. Patients received three courses of induction GIP (gemcitabine, ifosfamide, cisplatin). Non-progressing patients were randomised between three further courses of GIP or three courses of paclitaxel. Second-line paclitaxel was given to patients with primary failure (PF) to GIP and to those progressing after randomisation to further GIP (secondary failure or SF). One hundred sixty patients received second-line paclitaxel. Response rates were 7.7% for PF and 11.6% for SF (P=0.42). Median survival times (calculated from paclitaxel start) were 4.1 and 7.1 months for PF and SF (P=0.002). In multivariate analysis, three variables were independently associated with better survival: SF (hazard ratio (HR)=1.55, 95% confidence interval (CI) 1.08-2.22; P=0.02), normal haemoglobin level (HR=1.56, 95% CI 1.08-2.26; P=0.02) and minimal weight loss (HR=1.79, 95% CI 1.26-2.55; P=0.001). Paclitaxel in NSCLC patients, whether given for primary or for SF after cisplatin-based chemotherapy, demonstrates activity similar to other drugs considered active as second-line therapy.
Figures
References
-
- Barlesi F, Jacot W, Astoul P, Pujol JL (2006) Second-line treatment for advanced non-small cell lung cancer: a systematic review. Lung Cancer 51: 159–172 - PubMed
-
- Buccheri G, Ferrigno D (2004) Second-line weekly paclitaxel in patients with inoperable non-small cell lung cancer who fail combination chemotherapy with cisplatin. Lung Cancer 45: 227–236 - PubMed
-
- Camps C, Massuti B, Jimenez A, Maestu I, Gomez RG, Isla D, Gonzalez JL, Almenar D, Blasco A, Rosell R, Carrato A, Vinolas N, Batista N, Giron CG, Galan A, Lopez M, Blanco R, Provencio M, Diz P, Felip E (2006) Randomized phase III study of 3-weekly versus weekly docetaxel in pretreated advanced non-small-cell lung cancer: a Spanish Lung Cancer Group trial. Ann Oncol 17: 467–472 - PubMed
-
- Ceresoli GL, Gregorc V, Cordio S, Bencardino KB, Schipani S, Cozzarini C, Bordonaro R, Villa E (2004) Phase II study of weekly paclitaxel as second-line therapy in patients with advanced non-small cell lung cancer. Lung Cancer 44: 231–239 - PubMed
-
- Esteban E, Gonzalez de SL, Fernandez Y, Corral N, Fra J, Muniz I, Vieitez JM, Palacio I, Fernandez JL, Estrada E, Lacave AJ (2003) Prospective randomised phase II study of docetaxel versus paclitaxel administered weekly in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. Ann Oncol 14: 1640–1647 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical