Evolution of the core and pan-genome of Streptococcus: positive selection, recombination, and genome composition
- PMID: 17475002
- PMCID: PMC1929146
- DOI: 10.1186/gb-2007-8-5-r71
Evolution of the core and pan-genome of Streptococcus: positive selection, recombination, and genome composition
Abstract
Background: The genus Streptococcus is one of the most diverse and important human and agricultural pathogens. This study employs comparative evolutionary analyses of 26 Streptococcus genomes to yield an improved understanding of the relative roles of recombination and positive selection in pathogen adaptation to their hosts.
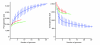
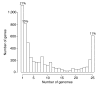
Results: Streptococcus genomes exhibit extreme levels of evolutionary plasticity, with high levels of gene gain and loss during species and strain evolution. S. agalactiae has a large pan-genome, with little recombination in its core-genome, while S. pyogenes has a smaller pan-genome and much more recombination of its core-genome, perhaps reflecting the greater habitat, and gene pool, diversity for S. agalactiae compared to S. pyogenes. Core-genome recombination was evident in all lineages (18% to 37% of the core-genome judged to be recombinant), while positive selection was mainly observed during species differentiation (from 11% to 34% of the core-genome). Positive selection pressure was unevenly distributed across lineages and biochemical main role categories. S. suis was the lineage with the greatest level of positive selection pressure, the largest number of unique loci selected, and the largest amount of gene gain and loss.
Conclusion: Recombination is an important evolutionary force in shaping Streptococcus genomes, not only in the acquisition of significant portions of the genome as lineage specific loci, but also in facilitating rapid evolution of the core-genome. Positive selection, although undoubtedly a slower process, has nonetheless played an important role in adaptation of the core-genome of different Streptococcus species to different hosts.
Figures

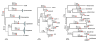

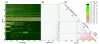



References
-
- Awadalla P. The evolutionary genomics of pathogen recombination. Nat Rev Genet. 2003;4:50–60. - PubMed
-
- Feil EJ, Holmes EC, Bessen DE, Chan MS, Day NP, Enright MC, Goldstein R, Hood DW, Kalia A, Moore CE, et al. Recombination within natural populations of pathogenic bacteria: short-term empirical estimates and long-term phylogenetic consequences. Proc Natl Acad Sci USA. 2001;98:182–187. - PMC - PubMed
-
- Spratt BG, Hanage WP, Feil EJ. The relative contributions of recombination and point mutation to the diversification of bacterial clones. Curr Opin Microbiol. 2001;4:602–606. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

