Strain differences in the disruption of prepulse inhibition of startle after systemic and intra-accumbens amphetamine administration
- PMID: 17475315
- PMCID: PMC1995415
- DOI: 10.1016/j.pbb.2007.03.014
Strain differences in the disruption of prepulse inhibition of startle after systemic and intra-accumbens amphetamine administration
Abstract
Background: Sprague Dawley (SD) rats are significantly more sensitive than Long Evans (LE) rats to the disruption of prepulse inhibition (PPI) by systemically-administered dopamine (DA) agonists. This strain difference is heritable and insensitive to cross-fostering. Inherited differences in the ability of elevated DA activity to disrupt PPI may be useful for understanding the neural basis for PPI deficits in schizophrenia and other neuropsychiatric disorders.
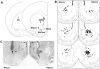
Methods: PPI was tested in male SD and LE rats after amphetamine (AMPH) was administered: 1) subcutaneously (sc), or intra-cerebrally (ic) into 2) the nucleus accumbens core (NACc; medial or lateral subregions) or the NAC shell; 3) the anteromedial striatum (AMS) or 4) the posterior striatum (PS).
Results: SD and LE rats had comparable PPI levels after sc vehicle injection. PPI was disrupted in SD but not LE rats after sc AMPH injection. LE insensitivity to AMPH was confirmed after sc injection into non-pigmented dermis, demonstrating that it did not reflect melanocyte sequestration of AMPH. PPI was also disrupted in SD rats after ic infusion into the NACc (medial core: p<0.005; lateral core: p<0.001); in LE rats, these effects only approached threshold levels (medial core: p<0.06; lateral core: p<0.051). In SD rats, the highest dose of AMPH (40 microg) tended to reduce PPI after infusion into the AMS or PS, while in LE rats, this dose potentiated PPI after PS infusion. Comparisons of PPI in SD vs. LE rats revealed significant main effects of strain (SD>LE) after vehicle infusions into the NACc subregions and the PS. Comparisons of pre-infusion "matching" data, data from the first infusion day, and data from separate rats in a "mock-infusion" paradigm is consistent with the possibility that SD>LE PPI after ic vehicle infusion reflects the impact of restraint stress on PPI in LE rats.
Conclusions: PPI is disrupted by AMPH administered sc or into the NACc in SD but not LE rats. Reduced PPI after ic vehicle infusion in LE vs. SD rats may reflect greater PPI-reducing effects of restraint stress in LE rats. The differential impact of restraint on PPI in SD vs. LE rats complicates the interpretation of strain differences in the effects of ic manipulations, but may provide an avenue for investigating the basis for differences in vulnerability to the gating-disruptive effects of stress.
Figures







Similar articles
-
Amphetamine effects on startle gating in normal women and female rats.Psychopharmacology (Berl). 2009 May;204(1):165-75. doi: 10.1007/s00213-008-1446-7. Epub 2009 Jan 16. Psychopharmacology (Berl). 2009. PMID: 19148623 Free PMC article.
-
Sensorimotor gating in rats is regulated by different dopamine-glutamate interactions in the nucleus accumbens core and shell subregions.Brain Res. 1996 May 25;722(1-2):168-76. doi: 10.1016/0006-8993(96)00209-0. Brain Res. 1996. PMID: 8813362
-
The effects of apomorphine and D-amphetamine on striatal c-Fos expression in Sprague-Dawley and Long Evans rats and their F1 progeny.Brain Res. 2006 Nov 13;1119(1):203-14. doi: 10.1016/j.brainres.2006.08.045. Epub 2006 Sep 18. Brain Res. 2006. PMID: 16979142
-
Amphetamine-modified acoustic startle responding and prepulse inhibition in adult and adolescent alcohol-preferring and -nonpreferring rats.Pharmacol Biochem Behav. 2003 Apr;75(1):163-71. doi: 10.1016/s0091-3057(03)00069-8. Pharmacol Biochem Behav. 2003. PMID: 12759124
-
Heritable differences in the dopaminergic regulation of behavior in rats: relationship to D2-like receptor G-protein function.Neuropsychopharmacology. 2006 Apr;31(4):721-9. doi: 10.1038/sj.npp.1300877. Neuropsychopharmacology. 2006. PMID: 16123742 Free PMC article.
Cited by
-
Schizophrenia-like reduced sensorimotor gating in intact inbred and outbred rats is associated with decreased medial prefrontal cortex activity and volume.Neuropsychopharmacology. 2019 Oct;44(11):1975-1984. doi: 10.1038/s41386-019-0392-x. Epub 2019 Apr 16. Neuropsychopharmacology. 2019. PMID: 30986819 Free PMC article.
-
Opposite effects of tolcapone on amphetamine-disrupted startle gating in low vs. high COMT-expressing rat strains.Pharmacol Biochem Behav. 2013 May;106:128-31. doi: 10.1016/j.pbb.2013.03.015. Epub 2013 Apr 6. Pharmacol Biochem Behav. 2013. PMID: 23567203 Free PMC article.
-
The effects of d-amphetamine, methylphenidate, sydnocarb, and caffeine on prepulse inhibition of the startle reflex in DBA/2 mice.Psychopharmacology (Berl). 2010 Aug;211(3):325-36. doi: 10.1007/s00213-010-1901-0. Epub 2010 Jun 12. Psychopharmacology (Berl). 2010. PMID: 20549488
-
1-Methyl-1,2,3,4-tetrahydroisoquinoline antagonizes a rise in brain dopamine metabolism, glutamate release in frontal cortex and locomotor hyperactivity produced by MK-801 but not the disruptions of prepulse inhibition, and impairment of working memory in rat.Neurotox Res. 2009 Nov;16(4):390-407. doi: 10.1007/s12640-009-9097-y. Epub 2009 Aug 1. Neurotox Res. 2009. PMID: 19649683
-
Short-term selective breeding for high and low prepulse inhibition of the acoustic startle response; pharmacological characterization and QTL mapping in the selected lines.Pharmacol Biochem Behav. 2008 Oct;90(4):525-33. doi: 10.1016/j.pbb.2008.04.004. Pharmacol Biochem Behav. 2008. PMID: 18513787 Free PMC article.
References
-
- Arguello PA, Gogos JA. Modeling Madness in Mice: One Piece at a Time. Neuron. 2006;52:179–196. - PubMed
-
- Bitsios P, Giakoumaki SG, Frangou S. The effects of dopamine agonists on prepulse inhibition in healthy men depend on baseline PPI values. Psychopharmacology. 2005;182:144–152. - PubMed
-
- Borges CR, Martin SD, Meyer LJ, Wilkins DG, Rollins DE. Influx and efflux of amphetamine and N-acetylamphetamine in keratinocytes, pigmented melanocytes, and nonpigmented melanocytes. J Pharm Sci. 2002;91:1523–1535. - PubMed
-
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. - PubMed
-
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

