The opioid antagonist, beta-funaltrexamine, inhibits chemokine expression in human astroglial cells
- PMID: 17475341
- PMCID: PMC1948894
- DOI: 10.1016/j.jneuroim.2007.03.021
The opioid antagonist, beta-funaltrexamine, inhibits chemokine expression in human astroglial cells
Abstract
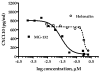
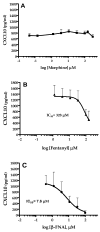
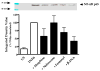
Emerging evidence indicates that neuroinflammatory responses in astroglia, including chemokine expression, are altered by opioids. Astroglial chemokines, such as CXCL10, are instrumental in response to many neuropathological insults. Opioid mediated disruption of astroglial CXCL10 expression may be detrimental in opioid abusers or patients receiving acute opioid therapy. We have characterized the in vitro effects of opioids on CXCL10 protein expression in human astroglial (A172) cells. The proinflammatory cytokine, tumor necrosis factor (TNF)alpha induced CXCL10 expression in A172 cells. Using MG-132, helenalin and SN50 [inhibitors of the transcription factor, nuclear factor (NF)-kappaB], we determined that NF-kappaB activation is instrumental in TNFalpha-induced CXCL10 expression in A172 astroglia. Morphine exposure during the 24 h TNFalpha stimulation period did not alter CXCL10 expression. However, fentanyl, a more potent mu-opioid receptor (MOR) agonist, inhibited TNFalpha-induced CXCL10 expression. Interestingly, neither the non-selective opioid receptor antagonist, naltrexone nor beta-funaltrexamine (beta-FNA), a highly selective MOR antagonist, blocked fentanyl mediated inhibition of TNFalpha-induced CXCL10 expression. Rather, beta-FNA dose-dependently inhibited TNFalpha-induced CXCL10 expression with a greater potency than that observed for fentanyl. Immunoblot analysis indicated that morphine, fentanyl and beta-FNA each reduced TNFalpha-induced nuclear translocation of NF-kappaB p65. These data show that beta-FNA and fentanyl inhibit TNFalpha-induced CXCL10 expression via a MOR-independent mechanism. Data also suggest that inhibition of TNFalpha-induced CXCL10 expression by fentanyl and beta-FNA is not directly related to a reduction in NF-kappaB p65 nuclear translocation. Further investigation is necessary in order to fully elucidate the mechanism through which these two opioid compounds inhibit CXCL10 expression. Understanding the mechanism by which chemokine expression is suppressed, particularly by the opioid antagonist, beta-FNA, may provide insights into the development of safe and effective treatments for neuroinflammation.
Figures




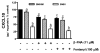
References
-
- Bajetto A, Bonavia R, Barbero S, Schettini G. J Neurochem. 2002;82:1311–1329. - PubMed
-
- Baumhaker Y, Gafni M, Keren O, Sarne Y. Mol Pharmacol. 1993;44:461–467. - PubMed
-
- Beech JS, Reckless J, Mosedale DE, Grainger DJ, Williams SC, Menon DK. J Cereb Blood Flow Metab. 2001;21:683–689. - PubMed
-
- Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. J Leukoc Biol. 2000;68:1–8. - PubMed
-
- Borner C, Kraus J, Schroder H, Ammer H, Hollt V. Mol Pharmacol. 2004;66:1719–1726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

