Ultracentrifugation of serum samples allows detection of hepatitis C virus RNA in patients with occult hepatitis C
- PMID: 17475654
- PMCID: PMC1933375
- DOI: 10.1128/JVI.02750-06
Ultracentrifugation of serum samples allows detection of hepatitis C virus RNA in patients with occult hepatitis C
Abstract
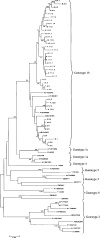
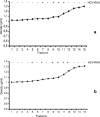
Occult hepatitis C virus (HCV) infection of patients with abnormal liver function tests of unknown origin who are anti-HCV and serum HCV RNA negative but who have HCV RNA in the liver has been described. As HCV replicates in the liver cells of these patients, it could be that the amount of circulating viral particles is under the detection limit of the most sensitive techniques. To prove this hypothesis, serum samples from 106 patients with occult HCV infection were analyzed. Two milliliters of serum was ultracentrifuged over a 10% sucrose cushion for 17 h at 100,000 x g(av), where av means average, and HCV RNA detection was performed by strand-specific real-time PCR. Out of the 106 patients, 62 (58.5%) had detectable serum HCV RNA levels after ultracentrifugation, with a median load of 70.5 copies/ml (range, 18 to 192). Iodixanol density gradient studies revealed that HCV RNA was positive at densities of 1.03 to 1.04 and from 1.08 to 1.19 g/ml, which were very similar to those found in the sera of patients with classical chronic HCV infection. Antigenomic HCV RNA was found in the livers of 56 of 62 (90.3%) patients with detectable serum HCV RNA levels after ultracentrifugation, compared to 27 of 44 (61.4%) negative patients (P < 0.001). No differences in the median loads of antigenomic HCV RNA between patients with an those without serum HCV RNA (4.5 x 10(4) [range, 7.9 x 10(2) to 1.0 x 10(6)] versus 2.3 x 10(4) [range, 4.0 x 10(2) to 2.2 x 10(5)]) were found. Alanine aminotransferase and gamma-glutamyl transpeptidase levels, liver necroinflammatory activity, and fibrosis did not differ between both groups. In conclusion, HCV RNA can be detected in the sera of patients with occult HCV infection after circulating viral particles are concentrated by ultracentrifugation.
Figures
Similar articles
-
Occult hepatitis C virus infection in patients in whom the etiology of persistently abnormal results of liver-function tests is unknown.J Infect Dis. 2004 Jan 1;189(1):7-14. doi: 10.1086/380202. Epub 2003 Dec 31. J Infect Dis. 2004. PMID: 14702147
-
Diagnosis of occult hepatitis C without the need for a liver biopsy.J Med Virol. 2010 Sep;82(9):1554-9. doi: 10.1002/jmv.21866. J Med Virol. 2010. PMID: 20648609
-
Comparative study on the clinical and virological characteristics among patients with single occult hepatitis B virus (HBV), single occult hepatitis C virus (HCV) and occult HBV and HCV dual infection.J Med Virol. 2007 Mar;79(3):236-41. doi: 10.1002/jmv.20784. J Med Virol. 2007. PMID: 17245725
-
Review: Occult hepatitis C virus infection: still remains a controversy.J Med Virol. 2014 Sep;86(9):1491-8. doi: 10.1002/jmv.23979. Epub 2014 Jun 4. J Med Virol. 2014. PMID: 24895180 Review.
-
Occult hepatitis B virus and hepatitis C virus infections.Rev Med Virol. 2008 May-Jun;18(3):139-57. doi: 10.1002/rmv.569. Rev Med Virol. 2008. PMID: 18265423 Review.
Cited by
-
Desperately seeking hepatitis C virus.World J Gastroenterol. 2008 May 14;14(18):2946-7. doi: 10.3748/wjg.14.2946. World J Gastroenterol. 2008. PMID: 18473430 Free PMC article.
-
Occult HCV Infection: The Current State of Knowledge.Iran Red Crescent Med J. 2015 Nov 29;17(11):e34181. doi: 10.5812/ircmj.34181. eCollection 2015 Nov. Iran Red Crescent Med J. 2015. PMID: 26734487 Free PMC article. Review.
-
A New Twist to a Chronic HCV Infection: Occult Hepatitis C.Gastroenterol Res Pract. 2015;2015:579147. doi: 10.1155/2015/579147. Epub 2015 Jun 24. Gastroenterol Res Pract. 2015. PMID: 26221136 Free PMC article. Review.
-
Hepatitis C virus infection in nephrology patients.J Nephropathol. 2013 Oct;2(4):217-33. doi: 10.12860/JNP.2013.36. Epub 2013 Jul 1. J Nephropathol. 2013. PMID: 24475454 Free PMC article. Review.
-
Prevalence and follow-up of occult HCV infection in an Italian population free of clinically detectable infectious liver disease.PLoS One. 2012;7(8):e43541. doi: 10.1371/journal.pone.0043541. Epub 2012 Aug 22. PLoS One. 2012. PMID: 22927986 Free PMC article.
References
-
- Bradley, D., K. McCaustland, K. Krawczynski, J. Spelbring, C. Humphrey, and E. H. Cook. 1991. Hepatitis C virus: buoyant density of the factor VIII-derived isolate in sucrose. J. Med. Virol. 34:206-208. - PubMed
-
- Bullock, G. C., D. E. Bruns, and D. M. Haverstick. 2002. Hepatitis C genotype determination by melting curve analysis with a single set of fluorescence resonance energy transfer probes. Clin. Chem. 48:2147-2154. - PubMed
-
- Castillo, I., M. Pardo, J. Bartolome, N. Ortiz-Movilla, E. Rodriguez-Iñigo, S. de Lucas, C. Salas, J. A. Jimenez-Heffernan, A. Perez-Mota, J. Graus, J. M. López-Alcorocho, and V. Carreño. 2004. Occult hepatitis C virus infection in patients in whom the etiology of persistently abnormal results of liver-function tests is unknown. J. Infect. Dis. 189:7-14. - PubMed
-
- Castillo, I., E. Rodríguez-Iñigo, J. M. López-Alcorocho, M. Pardo, J. Bartolomé, and V. Carreño. 2006. Hepatitis C virus replicates in the liver of patients who have a sustained response to antiviral treatment. Clin. Infect. Dis. 43:1277-1283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

