Tumor necrosis factor activates a conserved innate antiviral response to hepatitis B virus that destabilizes nucleocapsids and reduces nuclear viral DNA
- PMID: 17475655
- PMCID: PMC1933346
- DOI: 10.1128/JVI.00554-07
Tumor necrosis factor activates a conserved innate antiviral response to hepatitis B virus that destabilizes nucleocapsids and reduces nuclear viral DNA
Abstract
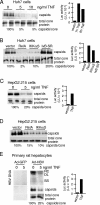
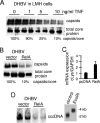
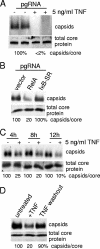
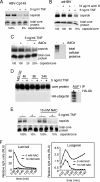
Tumor necrosis factor (TNF) is critical for the control of hepatitis B virus (HBV) in the clinical setting and in model systems. TNF induces noncytopathic suppression and clearance of HBV in animal models, possibly through reduction of viral nucleocapsids, but the mechanism is not well described. Here, we demonstrate the molecular mechanism and broad host range for TNF action against HBV. We show that TNF rapidly blocks HBV replication by promoting destabilization of preexisting cytoplasmic viral nucleocapsids containing viral RNA and DNA, as well as empty nucleocapsids. TNF destabilized human HBV nucleocapsids in a variety of human hepatocytic cell lines and in primary rat hepatocytes and also destabilized duck HBV (DHBV) nucleocapsids in chicken hepatocytic cells. Lysates from TNF-treated uninfected cells also destabilized HBV nucleocapsids in vitro. Moreover, inhibition of DHBV DNA replication by TNF blocks nuclear accumulation of the viral transcription template, maintenance of which is essential for the establishment and maintenance of chronic infection. We show that TNF destabilization of HBV nucleocapsids does not involve ubiquitination or methylation of the viral core protein and is not mediated by the nitric oxide free radical arm of the TNF pathway. These results define a novel antiviral mechanism mediated by TNF against multiple types of HBVs in different species.
Figures


Similar articles
-
Tumor necrosis factor alpha inhibition of hepatitis B virus replication involves disruption of capsid Integrity through activation of NF-kappaB.J Virol. 2003 Apr;77(7):4033-42. doi: 10.1128/jvi.77.7.4033-4042.2003. J Virol. 2003. PMID: 12634363 Free PMC article.
-
Hepatitis B Virus Covalently Closed Circular DNA Formation in Immortalized Mouse Hepatocytes Associated with Nucleocapsid Destabilization.J Virol. 2015 Sep;89(17):9021-8. doi: 10.1128/JVI.01261-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085156 Free PMC article.
-
Cleaved c-FLIP mediates the antiviral effect of TNF-α against hepatitis B virus by dysregulating hepatocyte nuclear factors.J Hepatol. 2016 Feb;64(2):268-277. doi: 10.1016/j.jhep.2015.09.012. Epub 2015 Sep 25. J Hepatol. 2016. PMID: 26409214
-
The innate immune response to hepatitis B virus infection: implications for pathogenesis and therapy.Antiviral Res. 2012 Dec;96(3):405-13. doi: 10.1016/j.antiviral.2012.10.001. Epub 2012 Oct 13. Antiviral Res. 2012. PMID: 23072881 Review.
-
Posttranscriptional control of HBV gene expression.Front Biosci. 2008 May 1;13:5533-47. doi: 10.2741/3097. Front Biosci. 2008. PMID: 18508603 Review.
Cited by
-
Adenoviral delivery of recombinant hepatitis B virus expressing foreign antigenic epitopes for immunotherapy of persistent viral infection.J Virol. 2014 Mar;88(5):3004-15. doi: 10.1128/JVI.02756-13. Epub 2013 Dec 26. J Virol. 2014. PMID: 24371056 Free PMC article.
-
Hepatitis B: future curative strategies.Curr Opin Infect Dis. 2014 Dec;27(6):528-34. doi: 10.1097/QCO.0000000000000110. Curr Opin Infect Dis. 2014. PMID: 25304392 Free PMC article. Review.
-
Hepatocyte nuclear factor 1α downregulates HBV gene expression and replication by activating the NF-κB signaling pathway.PLoS One. 2017 Mar 20;12(3):e0174017. doi: 10.1371/journal.pone.0174017. eCollection 2017. PLoS One. 2017. PMID: 28319127 Free PMC article.
-
Treatment of rheumatic diseases and hepatitis B virus coinfection.Rheumatol Int. 2015 Mar;35(3):385-92. doi: 10.1007/s00296-014-3195-8. Epub 2014 Dec 31. Rheumatol Int. 2015. PMID: 25549599 Free PMC article. Review.
-
Clinical Implications of Hepatitis B Virus RNA and Covalently Closed Circular DNA in Monitoring Patients with Chronic Hepatitis B Today with a Gaze into the Future: The Field Is Unprepared for a Sterilizing Cure.Genes (Basel). 2018 Oct 5;9(10):483. doi: 10.3390/genes9100483. Genes (Basel). 2018. PMID: 30301171 Free PMC article. Review.
References
-
- al-Wabel, A., M. al-Janadi, and S. Raziuddin. 1993. Cytokine profile of viral and autoimmune chronic active hepatitis. J. Allergy Clin. Immunol. 92:902-908. - PubMed
-
- Ando, K., L. G. Guidotti, S. Wirth, T. Ishikawa, G. Missale, T. Moriyama, R. D. Schreiber, H. J. Schlicht, S. N. Huang, and F. V. Chisari. 1994. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J. Immunol. 152:3245-3253. - PubMed
-
- Baron, J. L., L. Gardiner, S. Nishimura, K. Shinkai, R. Locksley, and D. Ganem. 2002. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity 16:583-594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

