The transmembrane domain of acid trehalase mediates ubiquitin-independent multivesicular body pathway sorting
- PMID: 17475771
- PMCID: PMC1924822
- DOI: 10.1091/mbc.e06-11-0995
The transmembrane domain of acid trehalase mediates ubiquitin-independent multivesicular body pathway sorting
Abstract
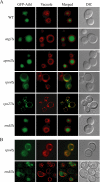
Trehalose serves as a storage source of carbon and plays important roles under various stress conditions. For example, in many organisms trehalose has a critical function in preserving membrane structure and fluidity during dehydration/rehydration. In the yeast Saccharomyces cerevisiae, trehalose accumulates in the cell when the nutrient supply is limited but is rapidly degraded when the supply of nutrients is renewed. Hydrolysis of trehalose in yeast depends on neutral trehalase and acid trehalase (Ath1). Ath1 resides and functions in the vacuole; however, it appears to catalyze the hydrolysis of extracellular trehalose. Little is known about the transport route of Ath1 to the vacuole or how it encounters its substrate. Here, through the use of various trafficking mutants we showed that this hydrolase reaches its final destination through the multivesicular body (MVB) pathway. In contrast to the vast majority of proteins sorted into this pathway, Ath1 does not require ubiquitination for proper localization. Mutagenesis analyses aimed at identifying the unknown targeting signal revealed that the transmembrane domain of Ath1 contains the information sufficient for its selective sequestration into MVB internal vesicles.
Figures



Similar articles
-
The Saccharomyces cerevisiae vacuolar acid trehalase is targeted at the cell surface for its physiological function.FEBS J. 2009 Oct;276(19):5432-46. doi: 10.1111/j.1742-4658.2009.07227.x. Epub 2009 Aug 24. FEBS J. 2009. PMID: 19703229
-
Evidence for a direct role of the Doa4 deubiquitinating enzyme in protein sorting into the MVB pathway.Traffic. 2007 May;8(5):566-81. doi: 10.1111/j.1600-0854.2007.00553.x. Epub 2007 Mar 21. Traffic. 2007. PMID: 17376168
-
Deletion of the ATH1 gene in Saccharomyces cerevisiae prevents growth on trehalose.FEBS Lett. 1996 May 20;386(2-3):235-8. doi: 10.1016/0014-5793(96)00450-4. FEBS Lett. 1996. PMID: 8647289
-
The ubiquitin code of yeast permease trafficking.Trends Cell Biol. 2010 Apr;20(4):196-204. doi: 10.1016/j.tcb.2010.01.004. Trends Cell Biol. 2010. PMID: 20138522 Review.
-
Acid trehalase in yeasts and filamentous fungi: localization, regulation and physiological function.FEMS Yeast Res. 2005 Apr;5(6-7):503-11. doi: 10.1016/j.femsyr.2005.01.002. FEMS Yeast Res. 2005. PMID: 15780651 Review.
Cited by
-
Suppressor of K+ transport growth defect 1 (SKD1) interacts with RING-type ubiquitin ligase and sucrose non-fermenting 1-related protein kinase (SnRK1) in the halophyte ice plant.J Exp Bot. 2013 May;64(8):2385-400. doi: 10.1093/jxb/ert097. Epub 2013 Apr 11. J Exp Bot. 2013. PMID: 23580756 Free PMC article.
-
Evaluating cellular roles and phenotypes associated with trehalose degradation genes in Saccharomyces cerevisiae.G3 (Bethesda). 2024 Nov 6;14(11):jkae215. doi: 10.1093/g3journal/jkae215. G3 (Bethesda). 2024. PMID: 39250759 Free PMC article.
-
Vacuolar hydrolysis and efflux: current knowledge and unanswered questions.Autophagy. 2019 Feb;15(2):212-227. doi: 10.1080/15548627.2018.1545821. Epub 2018 Nov 22. Autophagy. 2019. PMID: 30422029 Free PMC article. Review.
-
Mechanism of validamycin A inhibiting DON biosynthesis and synergizing with DMI fungicides against Fusarium graminearum.Mol Plant Pathol. 2021 Jul;22(7):769-785. doi: 10.1111/mpp.13060. Epub 2021 May 2. Mol Plant Pathol. 2021. PMID: 33934484 Free PMC article.
-
Anchors aweigh: protein localization and transport mediated by transmembrane domains.Trends Cell Biol. 2013 Oct;23(10):511-7. doi: 10.1016/j.tcb.2013.05.005. Epub 2013 Jun 24. Trends Cell Biol. 2013. PMID: 23806646 Free PMC article. Review.
References
-
- Alizadeh P., Klionsky D. J. Purification and biochemical characterization of the ATH1 gene product, vacuolar acid trehalase, from Saccharomyces cerevisiae. FEBS Lett. 1996;391:273–278. - PubMed
-
- Avaro S., Belgareh-Touze N., Sibella-Arguelles C., Volland C., Haguenauer-Tsapis R. Mutants defective in secretory/vacuolar pathways in the EUROFAN collection of yeast disruptants. Yeast. 2002;19:351–371. - PubMed
-
- Babst M. A protein's final ESCRT. Traffic. 2005;6:2–9. - PubMed
-
- Bonifacino J. S., Cosson P., Klausner R. D. Colocalized transmembrane determinants for ER degradation and subunit assembly explain the intracellular fate of TCR chains. Cell. 1990;63:503–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

