The transmembrane domain of acid trehalase mediates ubiquitin-independent multivesicular body pathway sorting
- PMID: 17475771
- PMCID: PMC1924822
- DOI: 10.1091/mbc.e06-11-0995
The transmembrane domain of acid trehalase mediates ubiquitin-independent multivesicular body pathway sorting
Abstract
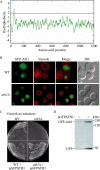
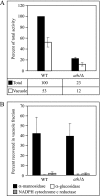
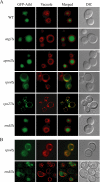
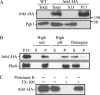
Trehalose serves as a storage source of carbon and plays important roles under various stress conditions. For example, in many organisms trehalose has a critical function in preserving membrane structure and fluidity during dehydration/rehydration. In the yeast Saccharomyces cerevisiae, trehalose accumulates in the cell when the nutrient supply is limited but is rapidly degraded when the supply of nutrients is renewed. Hydrolysis of trehalose in yeast depends on neutral trehalase and acid trehalase (Ath1). Ath1 resides and functions in the vacuole; however, it appears to catalyze the hydrolysis of extracellular trehalose. Little is known about the transport route of Ath1 to the vacuole or how it encounters its substrate. Here, through the use of various trafficking mutants we showed that this hydrolase reaches its final destination through the multivesicular body (MVB) pathway. In contrast to the vast majority of proteins sorted into this pathway, Ath1 does not require ubiquitination for proper localization. Mutagenesis analyses aimed at identifying the unknown targeting signal revealed that the transmembrane domain of Ath1 contains the information sufficient for its selective sequestration into MVB internal vesicles.
Figures



References
-
- Alizadeh P., Klionsky D. J. Purification and biochemical characterization of the ATH1 gene product, vacuolar acid trehalase, from Saccharomyces cerevisiae. FEBS Lett. 1996;391:273–278. - PubMed
-
- Avaro S., Belgareh-Touze N., Sibella-Arguelles C., Volland C., Haguenauer-Tsapis R. Mutants defective in secretory/vacuolar pathways in the EUROFAN collection of yeast disruptants. Yeast. 2002;19:351–371. - PubMed
-
- Babst M. A protein's final ESCRT. Traffic. 2005;6:2–9. - PubMed
-
- Bonifacino J. S., Cosson P., Klausner R. D. Colocalized transmembrane determinants for ER degradation and subunit assembly explain the intracellular fate of TCR chains. Cell. 1990;63:503–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

