The molecular mechanism of hepcidin-mediated ferroportin down-regulation
- PMID: 17475779
- PMCID: PMC1924807
- DOI: 10.1091/mbc.e07-01-0060
The molecular mechanism of hepcidin-mediated ferroportin down-regulation
Abstract
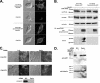
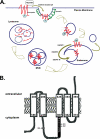
Ferroportin (Fpn) is the only known iron exporter in vertebrates. Hepcidin, a peptide secreted by the liver in response to iron or inflammation, binds to Fpn, inducing its internalization and degradation. We show that after binding of hepcidin, Fpn is tyrosine phosphorylated at the plasma membrane. Mutants of human Fpn that do not get internalized or that are internalized slowly show either absent or impaired phosphorylation. We identify adjacent tyrosines as the phosphorylation sites and show that mutation of both tyrosines prevents hepcidin-mediated Fpn internalization. Once internalized, Fpn is dephosphorylated and subsequently ubiquitinated. An inability to ubiquitinate Fpn does not prevent hepcidin-induced internalization, but it inhibits the degradation of Fpn. Ubiquitinated Fpn is trafficked through the multivesicular body pathway en route to degradation in the late endosome/lysosome. Depletion of proteins involved in multivesicular body trafficking (Endosome Sorting Complex Required for Transport proteins), by small-interfering RNA, reduces the trafficking of Fpn-green fluorescent to the lysosome.
Figures
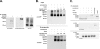




References
-
- Abboud S., Haile D. J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000;275:19906–19912. - PubMed
-
- Babst M., Katzmann D. J., Estepa-Sabal E. J., Meerloo T., Emr S. D. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 2002a;3:271–282. - PubMed
-
- Babst M., Katzmann D. J., Snyder W. B., Wendland B., Emr S. D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 2002b;3:283–289. - PubMed
-
- Babst M., Odorizzi G., Estepa E. J., Emr S. D. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

