Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection
- PMID: 17475862
- PMCID: PMC3777394
- DOI: 10.4049/jimmunol.178.10.6332
Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection
Abstract
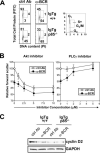
PI3K plays key roles in cell growth, differentiation, and survival by generating the second messenger phosphatidylinositol-(3,4,5)-trisphosphate (PIP3). PIP3 activates numerous enzymes, in part by recruiting them from the cytosol to the plasma membrane. We find that in immature B lymphocytes carrying a nonautoreactive Ag receptor, PI3K signaling suppresses RAG expression and promotes developmental progression. Inhibitors of PI3K signaling abrogate this positive selection. Furthermore, immature primary B cells from mice lacking the p85alpha regulatory subunit of PI3K suppress poorly RAG expression, undergo an exaggerated receptor editing response, and, as in BCR-ligated cells, fail to progress into the G1 phase of cell cycle. Moreover, immature B cells carrying an innocuous receptor have sustained elevation of PIP3 levels and activation of the downstream effectors phospholipase C (PLC)gamma2, Akt, and Bruton's tyrosine kinase. Of these, PLCgamma2 appears to play the most significant role in down-regulating RAG expression. It therefore appears that when the BCR of an immature B cell is ligated, PIP3 levels are reduced, PLCgamma2 activation is diminished, and receptor editing is promoted by sustained RAG expression. Taken together, our results provide evidence that PI3K signaling is an important cue required for fostering development of B cells carrying a useful BCR.
Figures




References
-
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. - PubMed
-
- Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igαβ heterodimer. Cell. 2004;117:787–800. - PubMed
-
- Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

