High levels of the BCR/ABL oncoprotein are required for the MAPK-hnRNP-E2 dependent suppression of C/EBPalpha-driven myeloid differentiation
- PMID: 17475908
- PMCID: PMC1924762
- DOI: 10.1182/blood-2007-03-078303
High levels of the BCR/ABL oncoprotein are required for the MAPK-hnRNP-E2 dependent suppression of C/EBPalpha-driven myeloid differentiation
Abstract
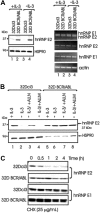
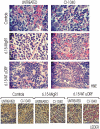
The inability of myeloid chronic myelogenous leukemia blast crisis (CML-BC) progenitors to undergo neutrophil differentiation depends on suppression of C/EBPalpha expression through the translation inhibitory activity of the RNA-binding protein hnRNP-E2. Here we show that "oncogene dosage" is a determinant factor for suppression of differentiation in CML-BC. In fact, high levels of p210-BCR/ABL are required for enhanced hnRNP-E2 expression, which depends on phosphorylation of hnRNP-E2 serines 173, 189, and 272 and threonine 213 by the BCR/ABL-activated MAPK(ERK1/2). Serine/threonine to alanine substitution abolishes hnRNP-E2 phosphorylation and markedly decreases its stability in BCR/ABL-expressing myeloid precursors. Similarly, pharmacologic inhibition of MAPK(ERK1/2) activity decreases hnRNP-E2 binding to the 5'UTR of C/EBPalpha mRNA by impairing hnRNP-E2 phosphorylation and stability. This, in turn, restores in vitro and/or in vivo C/EBPalpha expression and G-CSF-driven neutrophilic maturation of differentiation-arrested BCR/ABL(+) cell lines, primary CML-BC(CD34+) patient cells and lineage-negative mouse bone marrow cells expressing high levels of p210-BCR/ABL. Thus, increased BCR/ABL oncogenic tyrosine kinase activity is essential for suppression of myeloid differentiation of CML-BC progenitors as it is required for sustained activation of the MAPK(ERK1/2)-hnRNP-E2-C/EBPalpha differentiation-inhibitory pathway. Furthermore, these findings suggest the inclusion of clinically relevant MAPK inhibitors in the therapy of CML-BC.
Figures

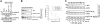



Similar articles
-
BCR-ABL suppresses C/EBPalpha expression through inhibitory action of hnRNP E2.Nat Genet. 2002 Jan;30(1):48-58. doi: 10.1038/ng791. Epub 2001 Dec 20. Nat Genet. 2002. PMID: 11753385
-
Identification of novel posttranscriptional targets of the BCR/ABL oncoprotein by ribonomics: requirement of E2F3 for BCR/ABL leukemogenesis.Blood. 2008 Jan 15;111(2):816-28. doi: 10.1182/blood-2007-05-090472. Epub 2007 Oct 9. Blood. 2008. PMID: 17925491 Free PMC article.
-
hnRNP A1 nucleocytoplasmic shuttling activity is required for normal myelopoiesis and BCR/ABL leukemogenesis.Mol Cell Biol. 2002 Apr;22(7):2255-66. doi: 10.1128/MCB.22.7.2255-2266.2002. Mol Cell Biol. 2002. PMID: 11884611 Free PMC article.
-
Leukemogenesis induced by wild-type and STI571-resistant BCR/ABL is potently suppressed by C/EBPalpha.Blood. 2006 Aug 15;108(4):1353-62. doi: 10.1182/blood-2006-01-011833. Epub 2006 May 2. Blood. 2006. PMID: 16670262 Free PMC article.
-
Regulation and deregulation of mRNA translation during myeloid maturation.Exp Hematol. 2011 Feb;39(2):133-41. doi: 10.1016/j.exphem.2010.10.011. Epub 2010 Nov 18. Exp Hematol. 2011. PMID: 21093533 Free PMC article. Review.
Cited by
-
Functions of heterogeneous nuclear ribonucleoproteins in stem cell potency and differentiation.Biomed Res Int. 2013;2013:623978. doi: 10.1155/2013/623978. Epub 2013 Jul 29. Biomed Res Int. 2013. PMID: 23984388 Free PMC article. Review.
-
Activity of the oral mitogen-activated protein kinase kinase inhibitor trametinib in RAS-mutant relapsed or refractory myeloid malignancies.Cancer. 2016 Jun 15;122(12):1871-9. doi: 10.1002/cncr.29986. Epub 2016 Mar 18. Cancer. 2016. PMID: 26990290 Free PMC article. Clinical Trial.
-
Leukemia stem cells: the root of chronic myeloid leukemia.Protein Cell. 2015 Jun;6(6):403-12. doi: 10.1007/s13238-015-0143-7. Epub 2015 Mar 10. Protein Cell. 2015. PMID: 25749979 Free PMC article. Review.
-
Crosstalk between hnRNP K and SET in ATRA-induced differentiation in acute promyelocytic leukemia.FEBS Open Bio. 2021 Jul;11(7):2019-2032. doi: 10.1002/2211-5463.13210. Epub 2021 Jun 17. FEBS Open Bio. 2021. PMID: 34058077 Free PMC article.
-
SRSF1 mediates cytokine-induced impaired imatinib sensitivity in chronic myeloid leukemia.Leukemia. 2020 Jul;34(7):1787-1798. doi: 10.1038/s41375-020-0732-1. Epub 2020 Feb 12. Leukemia. 2020. PMID: 32051529 Free PMC article.
References
-
- Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004;103:4010–4022. - PubMed
-
- Tenen DG, Hromas R, Licht JD, Zhang DE. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90:489–519. - PubMed
-
- Ward AC, Loeb DM, Soede-Bobok AA, Touw IP, Friedman AD. Regulation of granulopoiesis by transcription factors and cytokine signals. Leukemia. 2000;14:973–990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

