Immunologic evidence for lack of heterologous protection following resolution of HCV in patients with non-genotype 1 infection
- PMID: 17475911
- PMCID: PMC1975840
- DOI: 10.1182/blood-2007-01-069583
Immunologic evidence for lack of heterologous protection following resolution of HCV in patients with non-genotype 1 infection
Abstract
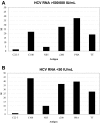
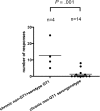
Chronic hepatitis C virus (HCV) infection is typically characterized by a lack of virus-specific CD4(+) T-cell-proliferative responses, but strong responses have been described in a subset of persons with persistent viremia. One possible explanation for these responses is that they were primed by an earlier resolved infection and do not recognize the current circulating virus. We defined all targeted epitopes using overlapping peptides corresponding to a genotype 1a strain in 44 patients chronically infected with different HCV genotypes (GT). Surprisingly, more HCV-specific CD4(+) T-cell responses were detected in patients with chronic non-GT1 infection compared with patients with chronic GT1 infection (P = .017). Notably, we found serologic evidence of a previous exposure to GT1 in 4 patients with non-GT1 infection, and these persons also demonstrated significantly more responses than non-GT1 patients in whom genotype and HCV serotype were identical (P < .001). Comparison of recognition of GT1-specific peptides to peptides representing autologous virus revealed the absence of cross-recognition of the autologous circulating virus. These data indicate that persistent HCV infection can occur in the presence of an HCV-specific T-cell response primed against a heterologous HCV strain, and suggest that clearance of 1 GT does not necessarily protect against subsequent exposure to a second GT.
Figures






Similar articles
-
Strain-specific T-cell suppression and protective immunity in patients with chronic hepatitis C virus infection.J Virol. 2005 Jun;79(11):6976-83. doi: 10.1128/JVI.79.11.6976-6983.2005. J Virol. 2005. PMID: 15890937 Free PMC article.
-
Characterization of novel HLA-DR11-restricted HCV epitopes reveals both qualitative and quantitative differences in HCV-specific CD4+ T cell responses in chronically infected and non-viremic patients.Eur J Immunol. 2001 May;31(5):1438-46. doi: 10.1002/1521-4141(200105)31:5<1438::AID-IMMU1438>3.0.CO;2-2. Eur J Immunol. 2001. PMID: 11465100
-
Differential virus-specific CD8+ T-cell epitope repertoire in hepatitis C virus genotype 1 versus 4.J Viral Hepat. 2018 Jul;25(7):779-790. doi: 10.1111/jvh.12874. Epub 2018 Feb 27. J Viral Hepat. 2018. PMID: 29397015
-
Subversion of immune responses by hepatitis C virus: immunomodulatory strategies beyond evasion?Curr Opin Immunol. 2003 Aug;15(4):443-9. doi: 10.1016/s0952-7915(03)00076-1. Curr Opin Immunol. 2003. PMID: 12900277 Review.
-
Virus-Specific Cellular Response in Hepatitis C Virus Infection.Arch Immunol Ther Exp (Warsz). 2016 Apr;64(2):101-10. doi: 10.1007/s00005-015-0364-8. Epub 2015 Oct 1. Arch Immunol Ther Exp (Warsz). 2016. PMID: 26429740 Review.
Cited by
-
Prospective follow-up of patients with acute hepatitis C virus infection in Brazil.Clin Infect Dis. 2010 May 1;50(9):1222-30. doi: 10.1086/651599. Clin Infect Dis. 2010. PMID: 20235831 Free PMC article.
-
The broad assessment of HCV genotypes 1 and 3 antigenic targets reveals limited cross-reactivity with implications for vaccine design.Gut. 2016 Jan;65(1):112-23. doi: 10.1136/gutjnl-2014-308724. Epub 2015 Jun 19. Gut. 2016. PMID: 26092843 Free PMC article.
-
Rapid early innate control of hepatitis C virus during IFN-α treatment compromises adaptive CD4+ T-cell immunity.Eur J Immunol. 2012 Sep;42(9):2383-94. doi: 10.1002/eji.201142072. Epub 2012 Aug 6. Eur J Immunol. 2012. PMID: 22653709 Free PMC article.
-
Comprehensive Review of Human Plasmodium falciparum-Specific CD8+ T Cell Epitopes.Front Immunol. 2019 Mar 21;10:397. doi: 10.3389/fimmu.2019.00397. eCollection 2019. Front Immunol. 2019. PMID: 30949162 Free PMC article. Review.
-
Adaptive immunity to the hepatitis C virus.Adv Virus Res. 2010;78:43-86. doi: 10.1016/B978-0-12-385032-4.00002-1. Adv Virus Res. 2010. PMID: 21040831 Free PMC article. Review.
References
-
- Gerlach JT, Diepolder HM, Zachoval R, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. - PubMed
-
- Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–1483. - PubMed
-
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. - PubMed
-
- Grebely J, Conway B, Raffa JD, Lai C, Krajden M, Tyndall MW. Hepatitis C virus reinfection in injection drug users. Hepatology. 2006;44:1139–1145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

