Negative BOLD fMRI response in the visual cortex carries precise stimulus-specific information
- PMID: 17476332
- PMCID: PMC1853239
- DOI: 10.1371/journal.pone.0000410
Negative BOLD fMRI response in the visual cortex carries precise stimulus-specific information
Abstract
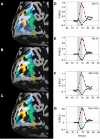
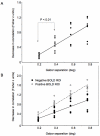
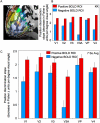
Sustained positive BOLD (blood oxygen level-dependent) activity is employed extensively in functional magnetic resonance imaging (fMRI) studies as evidence for task or stimulus-specific neural responses. However, the presence of sustained negative BOLD activity (i.e., sustained responses that are lower than the fixation baseline) has remained more difficult to interpret. Some studies suggest that it results from local "blood stealing" wherein blood is diverted to neurally active regions without a concomitant change of neural activity in the negative BOLD regions. However, other evidence suggests that negative BOLD is a result of local neural suppression. In both cases, regions of negative BOLD response are usually interpreted as carrying relatively little, if any, stimulus-specific information (hence the predominant reliance on positive BOLD activity in fMRI). Here we show that the negative BOLD response resulting from visual stimulation can carry high information content that is stimulus-specific. Using a general linear model (GLM), we contrasted standard flickering stimuli to a fixation baseline and found regions of the visual cortex that displayed a sustained negative BOLD response, consistent with several previous studies. Within these negative BOLD regions, we compared patterns of fMRI activity generated by flickering Gabors that were systematically shifted in position. As the Gabors were shifted further from each other, the correlation in the spatial pattern of activity across a population of voxels (such as the population of V1 voxels that displayed a negative BOLD response) decreased significantly. Despite the fact that the BOLD signal was significantly negative (lower than fixation baseline), these regions were able to discriminate objects separated by less than 0.5 deg (at approximately 10 deg eccentricity). The results suggest that meaningful, stimulus-specific processing occurs even in regions that display a strong negative BOLD response.
Conflict of interest statement
Figures


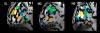



References
-
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. - PubMed
-
- Shulman GL. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. - PubMed
-
- Smith AT, Singh KD, Greenlee MW. Attentional suppression of activity in the human visual cortex. Neuroreport. 2000;11:271–277. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

