Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo
- PMID: 17476338
- PMCID: PMC1855077
- DOI: 10.1371/journal.pone.0000416
Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo
Abstract
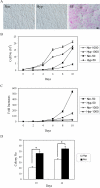
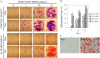
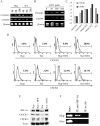
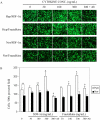
The ability of stem/progenitor cells to migrate and engraft into host tissues is key to their potential use in gene and cell therapy. Among the cells of interest are the adherent cells from bone marrow, referred to as mesenchymal stem cells or multipotent stromal cells (MSC). Since the bone marrow environment is hypoxic, with oxygen tensions ranging from 1% to 7%, we decided to test whether hypoxia can upregulate chemokine receptors and enhance the ability of human MSCs to engraft in vivo. Short-term exposure of MSCs to 1% oxygen increased expression of the chemokine receptors CX3CR1and CXCR4, both as mRNA and as protein. After 1-day exposure to low oxygen, MSCs increased in vitro migration in response to the fractalkine and SDF-1alpha in a dose dependent manner. Blocking antibodies for the chemokine receptors significantly decreased the migration. Xenotypic grafting into early chick embryos demonstrated cells from hypoxic cultures engrafted more efficiently than cells from normoxic cultures and generated a variety of cell types in host tissues. The results suggest that short-term culture of MSCs under hypoxic conditions may provide a general method of enhancing their engraftment in vivo into a variety of tissues.
Conflict of interest statement
Figures

References
-
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. - PubMed
-
- Matsuzaki Y, Kinjo K, Mulligan RC, Okano H. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity. 2004;20:87–93. - PubMed
-
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. - PubMed
-
- Chou SH, Kuo TK, Liu M, Lee OK. In utero transplantation of human bone marrow-derived multipotent mesenchymal stem cells in mice. J Orthop Res. 2006;24:301–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

