Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha
- PMID: 17476357
- PMCID: PMC1857264
- DOI: 10.1172/JCI29710
Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha
Abstract
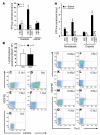
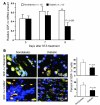
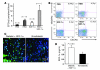
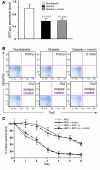
Endothelial progenitor cells (EPCs) are essential in vasculogenesis and wound healing, but their circulating and wound level numbers are decreased in diabetes. This study aimed to determine mechanisms responsible for the diabetic defect in circulating and wound EPCs. Since mobilization of BM EPCs occurs via eNOS activation, we hypothesized that eNOS activation is impaired in diabetes, which results in reduced EPC mobilization. Since hyperoxia activates NOS in other tissues, we investigated whether hyperoxia restores EPC mobilization in diabetic mice through BM NOS activation. Additionally, we studied the hypothesis that impaired EPC homing in diabetes is due to decreased wound level stromal cell-derived factor-1alpha (SDF-1alpha), a chemokine that mediates EPC recruitment in ischemia. Diabetic mice showed impaired phosphorylation of BM eNOS, decreased circulating EPCs, and diminished SDF-1alpha expression in cutaneous wounds. Hyperoxia increased BM NO and circulating EPCs, effects inhibited by the NOS inhibitor N-nitro-L-arginine-methyl ester. Administration of SDF-1alpha into wounds reversed the EPC homing impairment and, with hyperoxia, synergistically enhanced EPC mobilization, homing, and wound healing. Thus, hyperoxia reversed the diabetic defect in EPC mobilization, and SDF-1alpha reversed the diabetic defect in EPC homing. The targets identified, which we believe to be novel, can significantly advance the field of diabetic wound healing.
Figures



Comment in
-
Cellular and molecular basis of wound healing in diabetes.J Clin Invest. 2007 May;117(5):1219-22. doi: 10.1172/JCI32169. J Clin Invest. 2007. PMID: 17476353 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention. . Diabetes-related amputations of lower extremities in the Medicare population — Minnesota, 1993–1995. MMWR Morb. Mortal. Wkly. Rep. 1998;47:649–652. - PubMed
-
- Feener E.P., King G.L. Vascular dysfunction in diabetes mellitus. Lancet. 1997;350(Suppl. 1):SI9–SI13. - PubMed
-
- Phillips T.J. Chronic cutaneous ulcers: etiology and epidemiology. J. Invest. Dermatol. 1994;102:38S–41S. - PubMed
-
- Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. - PubMed
-
- Carmeliet P.2000Developmental biology. One cell, two fates. Nature. 40843 , 45 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

