Human frataxin: iron and ferrochelatase binding surface
- PMID: 17476391
- PMCID: PMC2862461
- DOI: 10.1039/b703195e
Human frataxin: iron and ferrochelatase binding surface
Abstract
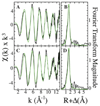
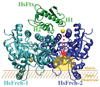
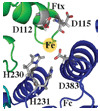
The coordinated iron structure and ferrochelatase binding surface of human frataxin have been characterized to provide insight into the protein's ability to serve as the iron chaperone during heme biosynthesis.
Figures

References
-
- Nair M, Adinolfi S, Pastore C, Kelly G, Temussi P, Pastore A. Structure (Cambridge, MA, U. S.) 2004;12:2037–2048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

