The in vitro effect of pH on osteoclasts and bone resorption in the cat: implications for the pathogenesis of FORL
- PMID: 17477347
- PMCID: PMC7167146
- DOI: 10.1002/jcp.21103
The in vitro effect of pH on osteoclasts and bone resorption in the cat: implications for the pathogenesis of FORL
Abstract
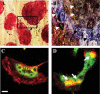
Dental disease due to osteoclast over-activity reaches epidemic proportions in older domestic cats and has also been reported in wild cats. Feline osteoclastic resorptive lesions (FORL) involve extensive resorption of the tooth leaving it liable to root fracture and subsequent tooth loss. The aetio-pathogenesis of FORL is not known. Recent work has shown that systemic acidosis causes increased osteoclast activation and that loci of infection or inflammation in cat mouth are likely to be acidotic. To investigate this, we generated osteoclasts from cat blood and found that they formed in large numbers (approximately 400) in cultures on bovine cortical bone slices. Acidosis caused an increase in the size of cells-in cultures maintained up to 14 days at basal pH 7.25, mean osteoclast area was 0.01 +/- 0.003 mm(2), whereas an 8.6-fold increase was observed in cells cultured between 11 and 14 days at pH 7.15 (0.086 +/- 0.004 mm(2)). Acidosis caused a modest increase in the number of osteoclasts. Exposure to pH 6.92 exhibited a 5-fold increase in the area of bone slices covered by resorption lacunae ( approximately 70% bone slice resorbed). In line with this finding, significant increases were observed in the expression of cathepsin K and proton pump enzymes (both approximately 3-fold) that are key enzymes reflective of resorptive activity in osteoclasts. These results demonstrate that acidosis is a major regulator of osteoclast formation and functional activation in the cat, and suggest that local pH changes may play a significant role in the pathogenesis of FORL.
(c) 2007 Wiley-Liss, Inc.
Figures



References
-
- Addison WC. 1978. Enzyme histochemical properties of kitten osteoclasts in bone imprint preparations. Histochem J 10: 645–656. - PubMed
-
- Addison WC. 1980. The effect of parathyroid hormone on the numbers of nuclei in feline odontoclasts in vivo. J Periodontal Res 15: 536–543. - PubMed
-
- Allen TD, Testa NG, Suda T, Schor SL, Onions D, Jarrett O, Boyde A. 1981. The production of putative osteoclasts in tissue culture—ultrastructure, formation and behaviour. Scan Electron Microsc 33: 347–354. - PubMed
-
- Arnett T. 2003. Regulation of bone cell function by acid‐base balance. Proc Nutr Soc 62: 511–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

