Sequences in attB that affect the ability of phiC31 integrase to synapse and to activate DNA cleavage
- PMID: 17478521
- PMCID: PMC1904298
- DOI: 10.1093/nar/gkm206
Sequences in attB that affect the ability of phiC31 integrase to synapse and to activate DNA cleavage
Abstract
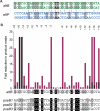
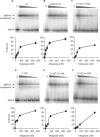
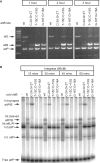
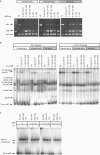
Phage integrases are required for recombination of the phage genome with the host chromosome either to establish or exit from the lysogenic state. C31 integrase is a member of the serine recombinase family of site-specific recombinases. In the absence of any accessory factors integrase is unidirectional, catalysing the integration reaction between the phage and host attachment sites, attP x attB to generate the hybrid sites, attL and attR. The basis for this directionality is due to selective synapsis of attP and attB sites. Here we show that mutations in attB can block the integration reaction at different stages. Mutations at positions distal to the crossover site inhibit recombination by destabilizing the synapse with attP without significantly affecting DNA-binding affinity. These data are consistent with the proposal that integrase adopts a specific conformation on binding to attB that permits synapsis with attP. Other attB mutants with changes close to the crossover site are able to form a stable synapse but cleavage of the substrates is prevented. These mutants indicate that there is a post-synaptic DNA recognition event that results in activation of DNA cleavage.
Figures



Similar articles
-
A motif in the C-terminal domain of phiC31 integrase controls the directionality of recombination.Nucleic Acids Res. 2008 Jul;36(12):3879-91. doi: 10.1093/nar/gkn269. Epub 2008 May 23. Nucleic Acids Res. 2008. PMID: 18502775 Free PMC article.
-
Control of directionality in the site-specific recombination system of the Streptomyces phage phiC31.Mol Microbiol. 2000 Oct;38(2):232-41. doi: 10.1046/j.1365-2958.2000.02142.x. Mol Microbiol. 2000. PMID: 11069650
-
Attachment site recognition and regulation of directionality by the serine integrases.Nucleic Acids Res. 2013 Sep;41(17):8341-56. doi: 10.1093/nar/gkt580. Epub 2013 Jul 2. Nucleic Acids Res. 2013. PMID: 23821671 Free PMC article.
-
Site-specific recombination by phiC31 integrase and other large serine recombinases.Biochem Soc Trans. 2010 Apr;38(2):388-94. doi: 10.1042/BST0380388. Biochem Soc Trans. 2010. PMID: 20298189 Review.
-
Phage-encoded Serine Integrases and Other Large Serine Recombinases.Microbiol Spectr. 2015 Aug;3(4). doi: 10.1128/microbiolspec.MDNA3-0059-2014. Microbiol Spectr. 2015. PMID: 26350324 Review.
Cited by
-
PhiC31 integrase interacts with TTRAP and inhibits NFkappaB activation.Mol Biol Rep. 2010 Jul;37(6):2809-16. doi: 10.1007/s11033-009-9829-3. Epub 2009 Sep 16. Mol Biol Rep. 2010. PMID: 19757154
-
A motif in the C-terminal domain of phiC31 integrase controls the directionality of recombination.Nucleic Acids Res. 2008 Jul;36(12):3879-91. doi: 10.1093/nar/gkn269. Epub 2008 May 23. Nucleic Acids Res. 2008. PMID: 18502775 Free PMC article.
-
Zinc is essential for high-affinity DNA binding and recombinase activity of ΦC31 integrase.Nucleic Acids Res. 2011 Aug;39(14):6137-47. doi: 10.1093/nar/gkr220. Epub 2011 Apr 20. Nucleic Acids Res. 2011. PMID: 21507889 Free PMC article.
-
Single-molecule analysis of ϕC31 integrase-mediated site-specific recombination by tethered particle motion.Nucleic Acids Res. 2016 Dec 15;44(22):10804-10823. doi: 10.1093/nar/gkw861. Epub 2016 Oct 5. Nucleic Acids Res. 2016. PMID: 27986956 Free PMC article.
-
Serine Integrase attP Binding and Specificity.J Mol Biol. 2018 Oct 19;430(21):4401-4418. doi: 10.1016/j.jmb.2018.09.007. Epub 2018 Sep 15. J Mol Biol. 2018. PMID: 30227134 Free PMC article.
References
-
- Belteki G, Gertsenstein M, Ow DW, Nagy A. Site-specific cassette exchange and germline transmission with mouse ES cells expressing ϕC31 integrase. Nat Biotechnol. 2003;21:321–324. - PubMed
-
- Khan MS, Khalid AM, KMalik KA. Phage ϕC31 integrase: a new tool in plastid genome engineering. Trends in Plant Science. 2005;10:1–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

