RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits
- PMID: 17478633
- PMCID: PMC1914118
- DOI: 10.1104/pp.107.100305
RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits
Abstract
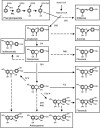
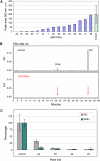
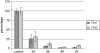
Parthenocarpy, the formation of seedless fruits in the absence of functional fertilization, is a desirable trait for several important crop plants, including tomato (Solanum lycopersicum). Seedless fruits can be of great value for consumers, the processing industry, and breeding companies. In this article, we propose a novel strategy to obtain parthenocarpic tomatoes by down-regulation of the flavonoid biosynthesis pathway using RNA interference (RNAi)-mediated suppression of chalcone synthase (CHS), the first gene in the flavonoid pathway. In CHS RNAi plants, total flavonoid levels, transcript levels of both Chs1 and Chs2, as well as CHS enzyme activity were reduced by up to a few percent of the corresponding wild-type values. Surprisingly, all strong Chs-silenced tomato lines developed parthenocarpic fruits. Although a relation between flavonoids and parthenocarpic fruit development has never been described, it is well known that flavonoids are essential for pollen development and pollen tube growth and, hence, play an essential role in plant reproduction. The observed parthenocarpic fruit development appeared to be pollination dependent, and Chs RNAi fruits displayed impaired pollen tube growth. Our results lead to novel insight in the mechanisms underlying parthenocarpic fruit development. The potential of this technology for applications in plant breeding and biotechnology will be discussed.
Figures




References
-
- Abad M, Monteiro AA (1989) The use of auxins for the production of greenhouse tomatoes in mild winter conditions: a review. Sci Hortic (Amsterdam) 38 167–192
-
- Bovy A, van den Berg C, de Vrieze G, Thompson WF, Weisbeek P, Smeekens S (1995) Light-regulated expression of the Arabidopsis thaliana ferredoxin gene requires sequences upstream and downstream of the transcription initiation site. Plant Mol Biol 27 27–39 - PubMed
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

