Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo
- PMID: 17479266
- PMCID: PMC11030170
- DOI: 10.1007/s00262-007-0330-3
Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo
Abstract
Background: We have treated three patients with carboxy-anhydrase-IX (CAIX) positive metastatic renal cell cancer (RCC) by adoptive transfer of autologous T-cells that had been gene-transduced to express a single-chain antibody-G250 chimeric receptor [scFv(G250)], and encountered liver toxicity necessitating adaptation of the treatment protocol. Here, we investigate whether or not the in vivo activity of the infused scFv(G250)(+) T cells is reflected by changes of selected immune parameters measured in peripheral blood.
Methods: ScFv(G250)-chimeric receptor-mediated functions of peripheral blood mononuclear cells (PBMC) obtained from three patients during and after treatment were compared to the same functions of scFv(G250)(+) T lymphocytes prior to infusion, and were correlated with plasma cytokine levels.
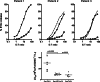
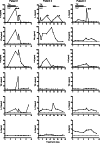
Results: Prior to infusion, scFv(G250)(+) T lymphocytes showed in vitro high levels of scFv(G250)-chimeric receptor-mediated functions such as killing of CAIX(+) RCC cell lines and cytokine production upon exposure to these cells. High levels of IFN-gamma were produced, whilst production of TNF-alpha, interleukin-4 (IL-4), IL-5 and IL-10 was variable and to lower levels, and that of IL-2 virtually absent. PBMC taken from patients during therapy showed lower levels of in vitro scFv(G250)-receptor-mediated functions as compared to pre-infusion, whilst IFN-gamma was the only detectable cytokine upon in vitro PBMC exposure to CAIX. During treatment, plasma levels of IFN-gamma increased only in the patient with the most prominent liver toxicity. IL-5 plasma levels increased transiently during treatment in all patients, which may have been triggered by the co-administration of IL-2.
Conclusion: ScFv(G250)-receptor-mediated functions of the scFv(G250)(+) T lymphocytes are, by and large, preserved in vivo upon administration, and may be reflected by fluctuations in plasma IFN-gamma levels.
Figures




References
-
- Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. - PubMed
-
- Lamers CHJ, Sleijfer S, Willemsen RA, Debets R, Kruit WHJ, Gratama JW, et al. Adoptive immuno-gene therapy of cancer with single chain antibody [scFv(Ig)] gene modified T lymphocytes. J Biol Regul Homeost Agents. 2004;18:134–140. - PubMed
-
- Weijtens ME, Willemsen RA, Valerio D, Stam K, Bolhuis RL. Single chain Ig/gamma gene-redirected human T lymphocytes produce cytokines, specifically lyse tumor cells, and recycle lytic capacity. J Immunol. 1996;157:836–843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

