Distribution and localization patterns of estrogen receptor-beta and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: localization of ERbeta and IGF-1R in substantia nigra
- PMID: 17480015
- PMCID: PMC2907103
- DOI: 10.1002/cne.21358
Distribution and localization patterns of estrogen receptor-beta and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: localization of ERbeta and IGF-1R in substantia nigra
Abstract
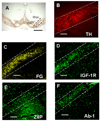
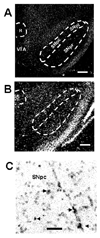
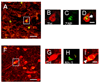
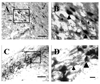
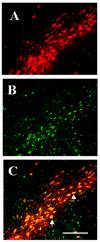
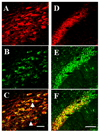
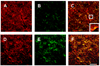
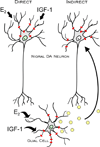
Although several studies have focused on the neuroprotective effects of estrogen (E2) on stroke, there have been tantalizing reports on the potential neuroprotective role of E2 in degenerative neuronal diseases such as Alzheimer's and Parkinson's (PD). In animal models of PD, E2 protects the nigrostriatal dopaminergic (DA) system against neurotoxins. However, little is known about the cellular and molecular mechanism(s) involved by which E2 elicits its neuroprotective effects on the nigrostriatal DA system. A preferred mechanism for neuroprotection is the interaction of E2 with specific neuroprotective growth factors and receptors. One such neuroprotective factor/receptor system is insulin-like growth factor-1 (IGF-1). E2 neuroprotective effects in the substantia nigra (SN) DA system have been shown to be dependent on IGF-1. To determine whether E2 also interacts with the IGF-1 receptor (IGF-1R) and to determine the cellular localization of estrogen receptor (ER) and IGF-1R, we compared the distribution of ER and IGF-1R in the SN. Stereological measurements revealed that 40% of the subpopulation of tyrosine hydroxylase-immunoreactive (TH-ir) SN pars compacta (SNpc) DA neurons are immunoreactive for estrogen receptor-beta (ERbeta). No immunolabeling for ERalpha was observed. In situ hybridization and immunocytochemistry studies confirmed the expression of IGF-1R mRNA and revealed that almost all TH-ir SNpc DA neurons were immunoreactive for IGF-1R, respectively. Moreover, one-third of glial fibrillary acidic protein (GFAP-ir) cells in the SN were ERbeta-ir, and 67% of GFAP-ir cells expressed IGF-1R-ir. Therefore, the localization of ERbeta and IGF-1R on SNpc DA neurons and astrocytes suggests a modulatory role of E2 on IGF-1R, and this modulation may affect neuroprotection.
Figures
Similar articles
-
PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson's disease.Dev Neurobiol. 2008 Apr;68(5):632-44. doi: 10.1002/dneu.20609. Dev Neurobiol. 2008. PMID: 18278798 Free PMC article.
-
Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions.J Neurosci Res. 2004 Jan 1;75(1):107-16. doi: 10.1002/jnr.10833. J Neurosci Res. 2004. PMID: 14689453
-
GPER and IGF-1R mediate the anti-inflammatory effect of genistein against lipopolysaccharide (LPS)-induced nigrostriatal injury in rats.J Steroid Biochem Mol Biol. 2021 Nov;214:105989. doi: 10.1016/j.jsbmb.2021.105989. Epub 2021 Aug 31. J Steroid Biochem Mol Biol. 2021. PMID: 34478828
-
Environment- and activity-dependent dopamine neurotransmitter plasticity in the adult substantia nigra.J Chem Neuroanat. 2016 Apr;73:21-32. doi: 10.1016/j.jchemneu.2015.12.009. Epub 2015 Dec 21. J Chem Neuroanat. 2016. PMID: 26718607 Review.
-
The extended amygdala and the dopamine system: another piece of the dopamine puzzle.J Neuropsychiatry Clin Neurosci. 2003 Summer;15(3):306-16. doi: 10.1176/jnp.15.3.306. J Neuropsychiatry Clin Neurosci. 2003. PMID: 12928506 Free PMC article. Review.
Cited by
-
Hormone replacement therapy and risk for neurodegenerative diseases.Int J Alzheimers Dis. 2012;2012:258454. doi: 10.1155/2012/258454. Epub 2012 Apr 4. Int J Alzheimers Dis. 2012. PMID: 22548198 Free PMC article.
-
Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and Neurodegeneration.Front Aging Neurosci. 2017 May 3;9:129. doi: 10.3389/fnagi.2017.00129. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28515690 Free PMC article. Review.
-
Neurodevelopmental effects of insulin-like growth factor signaling.Front Neuroendocrinol. 2012 Aug;33(3):230-51. doi: 10.1016/j.yfrne.2012.06.002. Epub 2012 Jun 16. Front Neuroendocrinol. 2012. PMID: 22710100 Free PMC article. Review.
-
The Neurosteroid Progesterone Underlies Estrogen Positive Feedback of the LH Surge.Front Endocrinol (Lausanne). 2011 Dec 2;2:90. doi: 10.3389/fendo.2011.00090. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22654832 Free PMC article.
-
Estradiol Uses Different Mechanisms in Astrocytes from the Hippocampus of Male and Female Rats to Protect against Damage Induced by Palmitic Acid.Front Mol Neurosci. 2017 Oct 24;10:330. doi: 10.3389/fnmol.2017.00330. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29114202 Free PMC article.
References
-
- Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999a;26:260–267. - PubMed
-
- Azcoitia I, Sierra A, Garcia-Segura LM. Neuroprotective effects of estradiol in the adult rat hippocampus: interaction with insulin-like growth factor-I signalling. J Neurosci Res. 1999b;58:815–822. - PubMed
-
- Bains M, Cousins JC, Roberts JL. Society for Neuroscience Abstract. Washington D.C: 2005. Estrogen Promotes Neuronal Survival in Mesencephalic Dopamine Progenitor Cultures.
-
- Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. Jama. 1991;265:1861–1867. - PubMed
-
- Blurton-Jones M, Tuszynski MH. Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J Comp Neurol. 2002;452:276–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

