Intramolecular Diels-Alder reactions of siloxacyclopentene constrained trienes
- PMID: 17480094
- PMCID: PMC2504012
- DOI: 10.1021/ol070858u
Intramolecular Diels-Alder reactions of siloxacyclopentene constrained trienes
Abstract
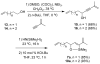
The synthesis of siloxacyclopentene-constrained trienes 7 and 10 and studies of their IMDA cycloadditions are described. The use of appropriately chosen thermal or Lewis acid conditions allows for cycloadducts 3-6 to be obtained with high levels of diastereoselectivity. These adducts possess trans-relationships between the hydroxyl group and the adjacent ring fusion proton, a stereochemical relationship not previously attainable in IMDA reactions.
Figures
Similar articles
-
Siloxacyclopentenes as dienophile-linked directing groups in intramolecular Diels-Alder reactions.Org Lett. 2008 Nov 20;10(22):5313-6. doi: 10.1021/ol802266s. Epub 2008 Oct 28. Org Lett. 2008. PMID: 18954061 Free PMC article.
-
Lewis acid catalyzed intramolecular Diels-Alder reactions of acyclic (Z)-substituted 1,3-dienes.Org Lett. 2001 Mar 22;3(6):957-60. doi: 10.1021/ol015667k. Org Lett. 2001. PMID: 11263925
-
Effect of Lewis acid catalysts on Diels-Alder and hetero-Diels-Alder cycloadditions sharing a common transition state.J Org Chem. 2008 Oct 3;73(19):7472-80. doi: 10.1021/jo801076t. Epub 2008 Sep 10. J Org Chem. 2008. PMID: 18781801
-
Recent Advances in Inverse-Electron-Demand Hetero-Diels-Alder Reactions of 1-Oxa-1,3-Butadienes.Top Curr Chem (Cham). 2016 Jun;374(3):24. doi: 10.1007/s41061-016-0026-2. Epub 2016 Apr 20. Top Curr Chem (Cham). 2016. PMID: 27573264 Free PMC article. Review.
-
Click Chemistry with Cyclopentadiene.Chem Rev. 2021 Jun 23;121(12):6777-6801. doi: 10.1021/acs.chemrev.0c01055. Epub 2021 Mar 2. Chem Rev. 2021. PMID: 33651602 Free PMC article. Review.
Cited by
-
Siloxacyclopentenes as dienophile-linked directing groups in intramolecular Diels-Alder reactions.Org Lett. 2008 Nov 20;10(22):5313-6. doi: 10.1021/ol802266s. Epub 2008 Oct 28. Org Lett. 2008. PMID: 18954061 Free PMC article.
-
Stereoselective Synthesis of the Decahydrofluorene Core of the Hirsutellones.Tetrahedron Lett. 2011 Apr 27;52(17):2072-2075. doi: 10.1016/j.tetlet.2010.10.111. Tetrahedron Lett. 2011. PMID: 21503271 Free PMC article.
-
Anion relay chemistry: access to the type II ARC reaction manifold through a fundamentally different reaction pathway exploiting 1-oxa-2-silacyclopentanes and related congeners.Angew Chem Int Ed Engl. 2011 Sep 12;50(38):8904-7. doi: 10.1002/anie.201103886. Epub 2011 Aug 10. Angew Chem Int Ed Engl. 2011. PMID: 21834108 Free PMC article. No abstract available.
References
-
- Ciganek B. Org. React. 1983;32:1.
-
- Craig D. Chem. Soc. Rev. 1986;16:187.
-
- Roush WR. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 5. Pergamon Press; Oxford: 1991. pp. 513–550.
-
- Takao K, Munakata R, Tadano K. Chem. Rev. 2005;105:4779. - PubMed
-
- Boeckman RK, Jr., Barta TE. J. Org. Chem. 1985;50:3421.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources