Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis
- PMID: 17480118
- PMCID: PMC1864991
- DOI: 10.1371/journal.ppat.0030062
Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis
Abstract
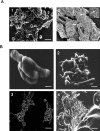
The role of biofilms in the pathogenesis of mycobacterial diseases remains largely unknown. Mycobacterium ulcerans, the etiological agent of Buruli ulcer, a disfiguring disease in humans, adopts a biofilm-like structure in vitro and in vivo, displaying an abundant extracellular matrix (ECM) that harbors vesicles. The composition and structure of the ECM differs from that of the classical matrix found in other bacterial biofilms. More than 80 proteins are present within this extracellular compartment and appear to be involved in stress responses, respiration, and intermediary metabolism. In addition to a large amount of carbohydrates and lipids, ECM is the reservoir of the polyketide toxin mycolactone, the sole virulence factor of M. ulcerans identified to date, and purified vesicles extracted from ECM are highly cytotoxic. ECM confers to the mycobacterium increased resistance to antimicrobial agents, and enhances colonization of insect vectors and mammalian hosts. The results of this study support a model whereby biofilm changes confer selective advantages to M. ulcerans in colonizing various ecological niches successfully, with repercussions for Buruli ulcer pathogenesis.
Conflict of interest statement
Figures




References
-
- Marsollier L, Aubry J, Saint-Andre JP, Robert R, Legras P, et al. Ecology and transmission of Mycobacterium ulcerans . Pathol Biol. 2003;51:490–495. - PubMed
-
- Portaels F, Chemlal K, Elsen P, Johnson PD, Hayman JA, et al. Mycobacterium ulcerans in wild animals. Rev Sci Tech Off int Epiz. 2001;20:252–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

