Changes in brain functional activation during resting and locomotor states after unilateral nigrostriatal damage in rats
- PMID: 17481921
- PMCID: PMC2039721
- DOI: 10.1016/j.neuroimage.2007.03.010
Changes in brain functional activation during resting and locomotor states after unilateral nigrostriatal damage in rats
Abstract
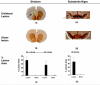
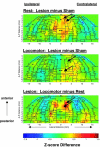
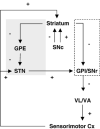
To evaluate functional neuronal compensation after partial damage to the nigrostriatal system, we lesioned rats unilaterally in the striatum with 6-hydroxydopamine. Five weeks later, cerebral perfusion was mapped at rest or during treadmill walking using [(14)C]-iodoantipyrine. Regional CBF-related tissue radioactivity (CBF-TR) was quantified by autoradiography and analyzed by statistical parametric mapping and region-of- interest analysis. Lesions were confirmed by tyrosine hydroxylase immunohistochemistry and changes in rotational locomotor activity. Functional compensations were bilateral and differed at rest and during treadmill walking. Consistent with the classic view of striatopallidal connections, CBF-TR of lesioned compared to sham-lesioned rats increased in the ipsilateral subthalamic nucleus (STN) and internal globus pallidus, and decreased in the striatum and external globus pallidus. Contrary to the classic view, CBF-TR increased in the ipsilateral ventral lateral, ventral anterior thalamus and motor cortex, as well as in the central medial thalamus, midline cerebellum, and contralateral STN. During walking, perfusion decreased in lesioned compared to sham-lesioned rats across the ipsilateral striato-pallidal-thalamic-cortical motor circuit. Compensatory increases were seen bilaterally in the ventromedial thalamus and red nucleus, in the contralateral STN, anterior substantia nigra, subiculum, motor cortex, and in midline cerebellum. Enhanced recruitment of associative sensory areas was noted cortically and subcortically. Future models of compensatory changes after nigrostriatal damage need to address the effects of increased neural activity by residual dopaminergic neurons, interhemispheric interactions and differences between resting and locomotor states. Identification of sites at which functional compensation occurs may define useful future targets for neurorehabilitative or neurorestorative interventions in Parkinson's disease.
Figures


Similar articles
-
Brain maps on the go: functional imaging during motor challenge in animals.Methods. 2008 Aug;45(4):255-61. doi: 10.1016/j.ymeth.2008.04.006. Epub 2008 Jun 11. Methods. 2008. PMID: 18554522 Free PMC article. Review.
-
Pirouetting pigs: A large non-primate animal model based on unilateral 6-hydroxydopamine lesioning of the nigrostriatal pathway.Brain Res Bull. 2018 May;139:167-173. doi: 10.1016/j.brainresbull.2018.02.010. Epub 2018 Feb 17. Brain Res Bull. 2018. PMID: 29462643
-
Morphological and biochemical adaptations to unilateral dopamine denervation of the neostriatum in newborn rats.Neuroscience. 1997 Apr;77(3):753-66. doi: 10.1016/s0306-4522(96)00500-3. Neuroscience. 1997. PMID: 9070750
-
Neuronal activity in the medial associative-limbic and lateral motor part of the rat subthalamic nucleus and the effect of 6-hydroxydopamine-induced lesions of the dorsolateral striatum.J Comp Neurol. 2013 Oct 1;521(14):3226-40. doi: 10.1002/cne.23342. J Comp Neurol. 2013. PMID: 23787707
-
Remote brain network changes after unilateral cortical impact injury and their modulation by acetylcholinesterase inhibition.J Neurotrauma. 2013 Jun 1;30(11):907-19. doi: 10.1089/neu.2012.2657. J Neurotrauma. 2013. PMID: 23343118 Free PMC article.
Cited by
-
Cerebellar Metabolic Connectivity during Treadmill Walking before and after Unilateral Dopamine Depletion in Rats.Int J Mol Sci. 2024 Aug 7;25(16):8617. doi: 10.3390/ijms25168617. Int J Mol Sci. 2024. PMID: 39201305 Free PMC article.
-
Lesion of the cerebellar noradrenergic innervation enhances the harmaline-induced tremor in rats.Cerebellum. 2011 Jun;10(2):267-80. doi: 10.1007/s12311-011-0250-9. Cerebellum. 2011. PMID: 21279489 Free PMC article.
-
Brain maps on the go: functional imaging during motor challenge in animals.Methods. 2008 Aug;45(4):255-61. doi: 10.1016/j.ymeth.2008.04.006. Epub 2008 Jun 11. Methods. 2008. PMID: 18554522 Free PMC article. Review.
-
The cerebellum in Parkinson's disease.Brain. 2013 Mar;136(Pt 3):696-709. doi: 10.1093/brain/aws360. Epub 2013 Feb 11. Brain. 2013. PMID: 23404337 Free PMC article. Review.
-
Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease.Lancet Neurol. 2013 Jul;12(7):716-26. doi: 10.1016/S1474-4422(13)70123-6. Lancet Neurol. 2013. PMID: 23769598 Free PMC article.
References
-
- Albani G, Kunig G, Soelch CM, Mauro A, Priano L, Martignoni E, Leenders KL. The role of language areas in motor control dysfunction in Parkinson’s disease. Neurol. Sci. 2001;22:43–44. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res. 1990;85:119–146. - PubMed
-
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

