Influence of anisotropic conduction properties in the propagation of the cardiac action potential
- PMID: 17482242
- PMCID: PMC1995420
- DOI: 10.1016/j.pbiomolbio.2007.03.014
Influence of anisotropic conduction properties in the propagation of the cardiac action potential
Abstract
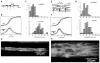
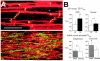
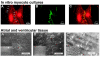
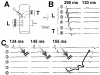
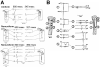
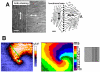
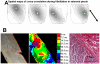
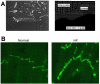
Anisotropy, the property of being directionally dependent, is ubiquitous in nature. Propagation of the electrical impulse in cardiac tissue is anisotropic, a property that is determined by molecular, cellular, and histological determinants. The properties and spatial arrangement of connexin molecules, the cell size and geometry, and the fiber orientation and arrangement are examples of structural determinants of anisotropy. Anisotropy is not a static property but is subject to dynamic functional regulation, mediated by modulation of gap junctional conductance. Tissue repolarization is also anisotropic. The relevance of anisotropy extends beyond normal propagation and has important implications in pathological states, as a potential substrate for abnormal rhythms and reentry.
Figures











References
-
- Abildskov JA. Effects of activation sequence on the local recovery of ventricular excitability in the dog. Circ Res. 1976;38:240–3. - PubMed
-
- Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2004;95:717–25. - PubMed
-
- Akar FG, Tomaselli GF. Conduction abnormalities in nonischemic dilated cardiomyopathy: basic mechanisms and arrhythmic consequences. Trends Cardiovasc Med. 2005;15:259–64. - PubMed
-
- Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. II. The role of nonuniform recovery of excitability in the occurrence of unidirectional block, as studied with multiple microelectrodes. Circ Res. 1976;39:168–77. - PubMed
-
- Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977;41:9–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

