Responses of the mitochondrial alpha-ketoglutarate dehydrogenase complex to thiamine deficiency may contribute to regional selective vulnerability
- PMID: 17482317
- PMCID: PMC2753422
- DOI: 10.1016/j.neuint.2007.03.010
Responses of the mitochondrial alpha-ketoglutarate dehydrogenase complex to thiamine deficiency may contribute to regional selective vulnerability
Abstract
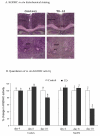
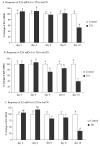
Thiamine-dependent enzymes are diminished in multiple neurodegenerative diseases. Thiamine deficiency (TD) reduces the activity of thiamine dependent-enzymes [e.g., the alpha-ketoglutarate dehydrogenase complex (KGDHC)], induces regional selective neurodegeneration and serves as a model of a mild impairment of oxidative metabolism. The current experiments tested whether changes in KGDHC protein subunits (E1k, E2k and E3) or activity or message levels underlie the selective loss of neurons in particular brain regions. Thus, TD-induced changes in these variables in the brain region most vulnerable to TD [the sub-medial thalamic nucleus (SmTN)] were compared to those in a region that is relatively resistant to TD (cortex) at stages of TD when the neuron loss in SmTN is not present, minimal or severe. Impaired motor performance on rotarod was apparent by 8 days of TD (-32%) and was severe by 10 days of TD (-97%). At TD10, the overall KGDHC activity measured by an in situ histochemical staining method declined 52% in SmTN but only 20% in cortex. Reductions in the E2k and E3 mRNA in SmTN occurred as early as TD6 (-28 and -18%, respectively) and were more severe by TD10 (-61 and -66%, respectively). On the other hand, the level of E1k mRNA did not decline in SmTN until TD10 (-48%). In contrast, TD did not alter mRNA levels of the subunits in cortex at late stages. Western blots and immunocytochemistry revealed different aspects of the changes in protein levels. In SmTN, the immunoreactivity of E1k and E3 by Western blotting increased 34 and 40%, respectively, only at TD8. In cortex, the immunoreactivity of the three subunits was not altered. Immunocytochemical staining of brain sections from TD10 mice indicated a reduction in the immunoreactivity of all subunits in SmTN, but not in cortex. These findings demonstrate that the response of the KGDHC activity, mRNA and immunoreactivity of E1k, E2k and E3 to TD is region and time dependent. Loss of KGDHC activity in cortex is likely related to post-translational modification rather than a loss of protein, whereas in SmTN transcriptional and post-translational modifications may account for diminished KGDHC activity. Moreover, the earlier detection in TD induced-changes of the transcripts of KGDHC indicates that transcriptional modification of the two subunits (E2k and E3) of KGDHC may be one of the early events in the cascade leading to selective neuronal death.
Figures





References
-
- Albers DS, Augood SJ, Park LC, Browne SE, Martin DM, Adamson J, Hutton M, Standaert DG, Vonsattel JP, Gibson GE, Beal MF. Frontal lobe dysfunction in progressive supranuclear palsy: evidence for oxidative stress and mitochondrial impairment. J Neurochem. 2000;74:878–81. - PubMed
-
- Barclay LL, Gibson GE, Blass JP. Impairment of behavior and acetylcholine metabolism in thiamine deficiency. J Pharmacol Exp Ther. 1981;217:537–43. - PubMed
-
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–71. - PubMed
-
- Bubber P, Ke ZJ, Gibson GE. Tricarboxylic acid cycle enzymes following thiamine deficiency. Neurochem. Int. 2004;45:1021–8. - PubMed
-
- Butterworth RF, Giguere JF, Besnard AM. Activities of thiamine-dependent enzymes in two experimental models of thiamine-deficiency encephalopathy. 2. alpha-Ketoglutarate dehydrogenase. Neurochem. Res. 1986;11:567–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

