The principal neuronal gD-type 3-O-sulfotransferases and their products in central and peripheral nervous system tissues
- PMID: 17482450
- PMCID: PMC1993827
- DOI: 10.1016/j.matbio.2007.03.002
The principal neuronal gD-type 3-O-sulfotransferases and their products in central and peripheral nervous system tissues
Abstract
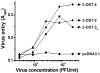
Within the nervous system, heparan sulfate (HS) of the cell surface and extracellular matrix influences developmental, physiologic and pathologic processes. HS is a functionally diverse polysaccharide that employs motifs of sulfate groups to selectively bind and modulate various effector proteins. Specific HS activities are modulated by 3-O-sulfated glucosamine residues, which are generated by a family of seven 3-O-sulfotransferases (3-OSTs). Most isoforms we herein designate as gD-type 3-OSTs because they generate HS(gD+), 3-O-sulfated motifs that bind the gD envelope protein of herpes simplex virus 1 (HSV-1) and thereby mediate viral cellular entry. Certain gD-type isoforms are anticipated to modulate neurobiologic events because a Drosophila gD-type 3-OST is essential for a conserved neurogenic signaling pathway regulated by Notch. Information about 3-OST isoforms expressed in the nervous system of mammals is incomplete. Here, we identify the 3-OST isoforms having properties compatible with their participation in neurobiologic events. We show that 3-OST-2 and 3-OST-4 are principal isoforms of brain. We find these are gD-type enzymes, as they produce products similar to a prototypical gD-type isoform, and they can modify HS to generate receptors for HSV-1 entry into cells. Therefore, 3-OST-2 and 3-OST-4 catalyze modifications similar or identical to those made by the Drosophila gD-type 3-OST that has a role in regulating Notch signaling. We also find that 3-OST-2 and 3-OST-4 are the predominant isoforms expressed in neurons of the trigeminal ganglion, and 3-OST-2/4-type 3-O-sulfated residues occur in this ganglion and in select brain regions. Thus, 3-OST-2 and 3-OST-4 are the major neural gD-type 3-OSTs, and so are prime candidates for participating in HS-dependent neurobiologic events.
Figures






References
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Borjigin J, Deng J, Sun X, De Jesus M, Liu T, Wang MM. Diurnal pineal 3-O-sulphotransferase 2 expression controlled by beta-adrenergic repression. J Biol Chem. 2003;278:16315–16319. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

