Role of erythropoietin in the brain
- PMID: 17482474
- PMCID: PMC2083122
- DOI: 10.1016/j.critrevonc.2007.03.001
Role of erythropoietin in the brain
Abstract
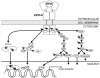
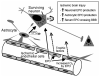
Multi-tissue erythropoietin receptor (EPO-R) expression provides for erythropoietin (EPO) activity beyond its known regulation of red blood cell production. This review highlights the role of EPO and EPO-R in brain development and neuroprotection. EPO-R brain expression includes neural progenitor cells (NPC), neurons, glial cells and endothelial cells. EPO is produced in brain in a hypoxia sensitive manner, stimulates NPC proliferation and differentiation, and neuron survival, and contributes to ischemic preconditioning. Mice lacking EPO or EPO-R exhibit increased neural cell apoptosis during development before embryonic death due to severe anemia. EPO administration provides neural protection in animal models of brain ischemia and trauma, reducing the extent of injury and damage. Intrinsic EPO production in brain and EPO stimulation of endothelial cells contribute to neuroprotection and these are of particular importance since only low levels of EPO appear to cross the blood-brain barrier when administered at high dose intravenously. The therapeutic potential of EPO for brain ischemia/trauma and neurodegenerative diseases has shown promise in early clinical trial and awaits further validation.
Figures

References
-
- Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76(1):105–16. - PubMed
-
- Jelkmann W, Wagner K. Beneficial and ominous aspects of the pleiotropic action of erythropoietin. Ann Hematol. 2004;83(11):673–86. - PubMed
-
- Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83(1):59–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

